FDA Clears Enspectra’s VIO System for Biopsy-free Skin Imaging
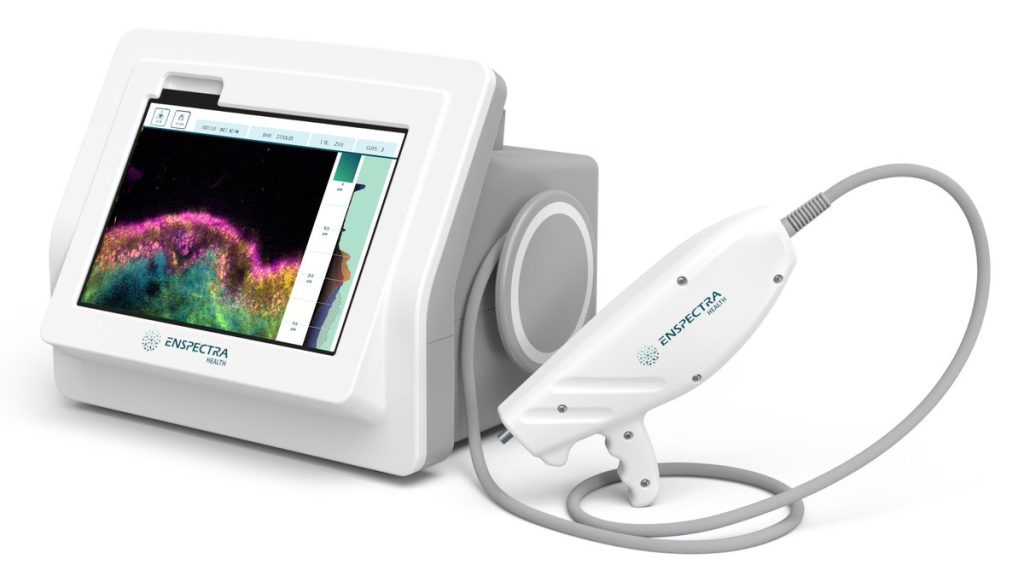
The U.S Food and Drug Administration (FDA) has granted 510(k) marketing clearance to Enspectra Health’s VIO System, a first-of-its-kind technology that combines reflectance confocal and multiphoton microscopy to enable cellular resolution, and cross-sectional imaging of skin in seconds. Vio is portable, handheld, and digitizes pathology directly from a patient’s skin to provide clinical insights for […]
