Dermavant Submits Supplemental sNDA to FDA for VTAMA Cream for AD in Adults and Children 2 Years of Age and Older
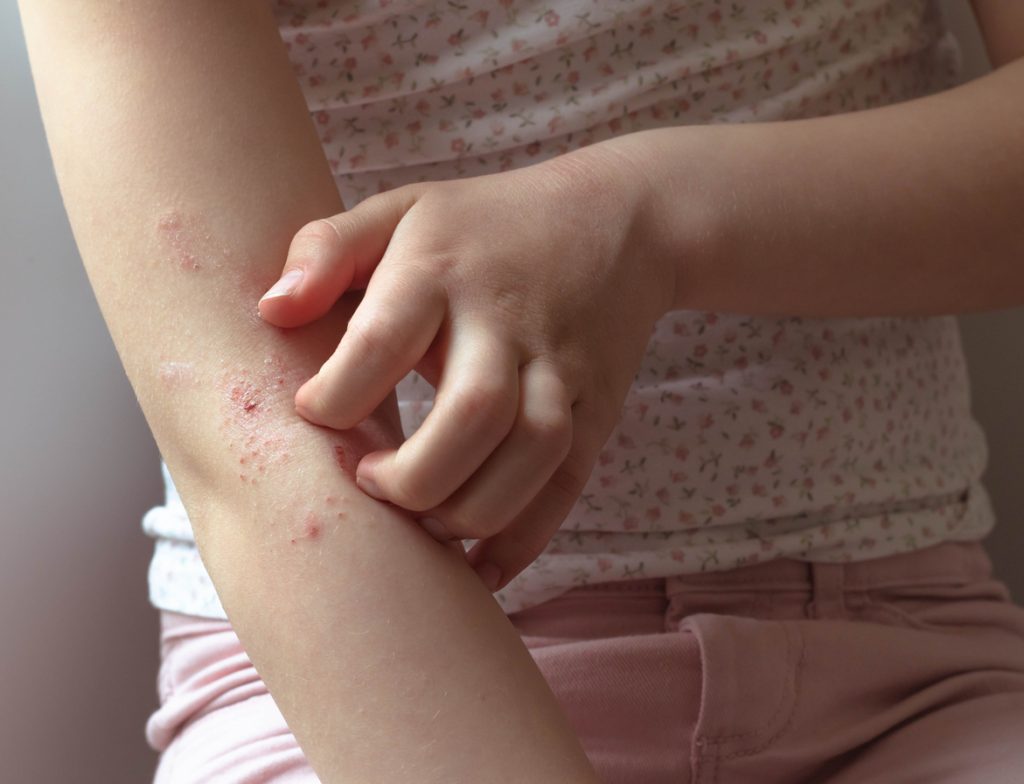
Dermavant Sciences has submitted a Supplemental New Drug Application (sNDA) to the U.S. Food and Drug Administration (FDA) for VTAMA (tapinarof) cream, 1% for the topical treatment of atopic dermatitis (AD) in adults and children 2 years of age and older. “We are confident, that VTAMA cream, if approved by the FDA, will be well […]
