Breaking News: Japan First in the World to Approve Dupilumab for CSU
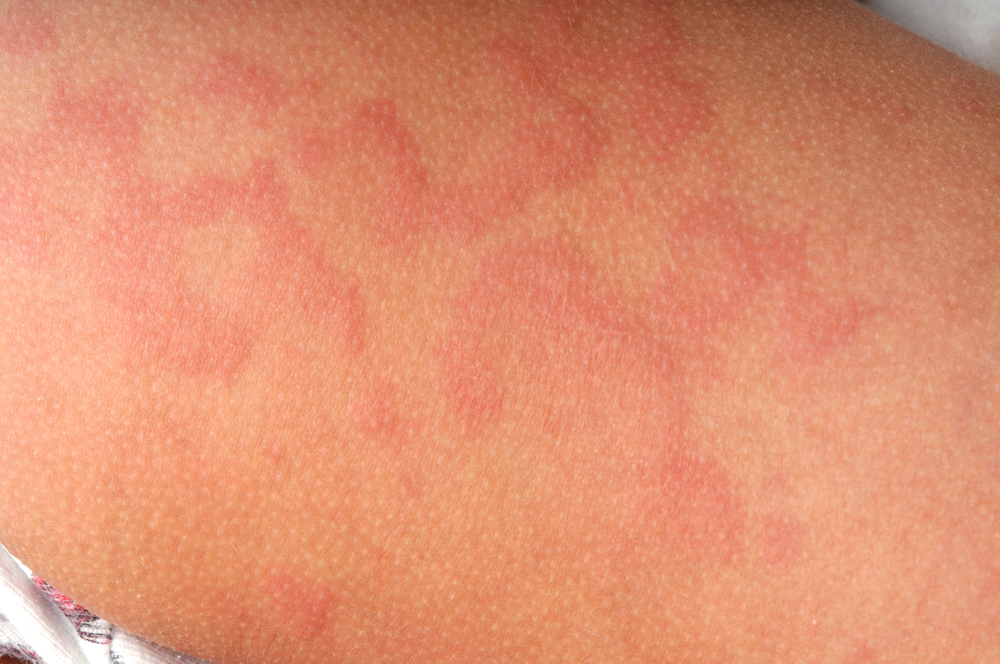
The Ministry of Health, Labor and Welfare (MHLW) in Japan has granted marketing and manufacturing authorization for dupilumab (Dupixent, Sanofi and Regeneron Pharmaceuticals, Inc.) for the treatment of chronic spontaneous urticaria (CSU) in people aged 12 years and older whose disease is not adequately controlled with existing therapy. Japan is the first country to approve dupilumab […]
