FDA Approves New Nonsteroidal Topical for AD: All About Tapinarof Cream, 1% (Vtama, Organon)
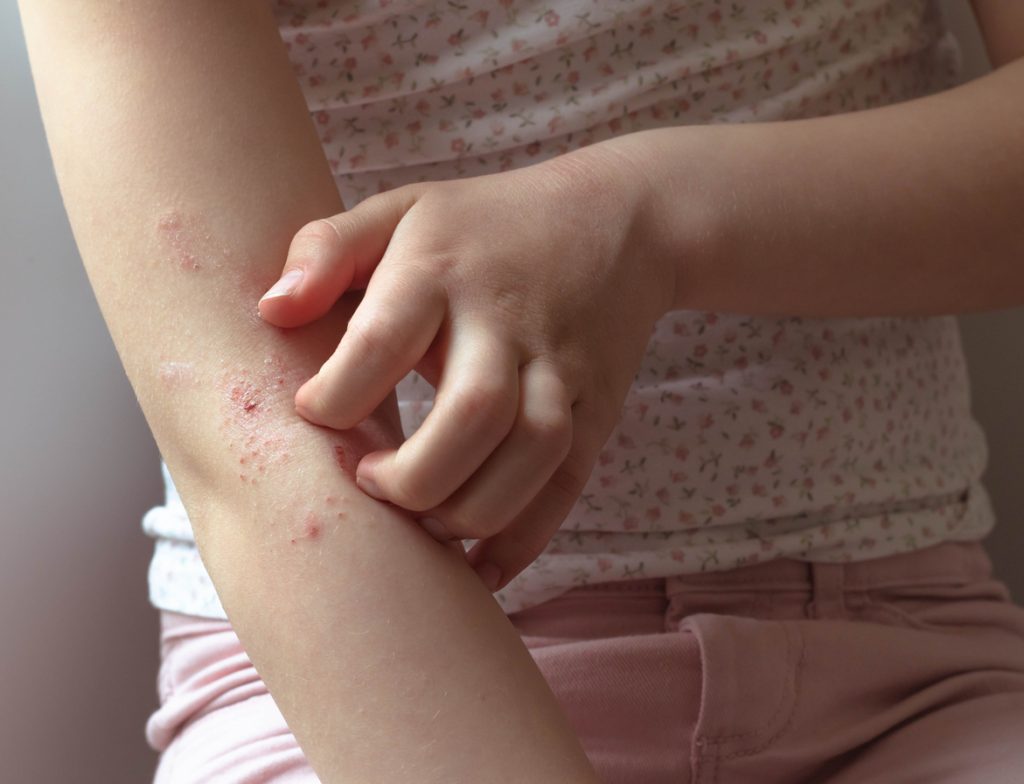
The U.S. Food and Drug Administration (FDA) has approved tapinarof cream, 1% (Vtama, Organon) for atopic dermatitis (AD) in adults and pediatric patients aged 2 and up. Tapinarof cream, 1% is a novel aryl hydrocarbon reception agonist that can be used once daily in patients with mild, moderate, and severe disease as well as those […]
