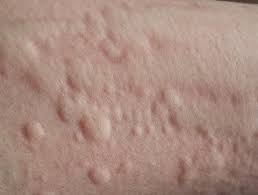
Patient enrollment is now complete in Celldex’s Phase 2 clinical study of barzolvolimab for the treatment of the two most common forms of chronic inducible urticaria (CIndU)—cold urticaria (ColdU) and symptomatic dermographism (SD).
Mast cell activation is known to be a critical driver in ColdU and SD, and barzolvolimab is a humanized monoclonal antibody that specifically binds the receptor tyrosine kinase KIT with high specificity and potently inhibits its activity, which is required for mast cell function and survival.
The randomized, double-blind, placebo-controlled, parallel group Phase 2 study is evaluating the efficacy and safety profile of multiple dose regimens of barzolvolimab in patients with CIndU who remain symptomatic despite antihistamine therapy, to determine the optimal dosing strategy.
For the study, 196 patients in two cohorts (differentiated by CIndU subtype) including 97 patients with ColdU and 99 patients with SD were randomly assigned on a 1:1:1 ratio to receive subcutaneous injections of barzolvolimab at 150 mg every 4 weeks, 300 mg every 8 weeks or placebo during a 20-week treatment phase. Patients then enter a follow-up phase for an additional 24 weeks. The primary endpoint of the study is the percentage of patients with a negative provocation test at Week 12 (using TempTest for ColdU and FricTest for SD). Secondary endpoints include safety and other assessments of clinical activity including CTT (critical temperature threshold), CFT (critical friction threshold), and WI-NRS (worst itch numeric rating scale).
“While individuals with inducible urticaria go to great lengths to avoid disease triggers in their daily lives, many find it impossible to do so and are severely burdened by this disease,” says Anthony Marucci, President and Chief Executive Officer of Celldex Therapeutics, in a news release. “This is compounded by the fact that there are currently no approved therapies for CIndU other than antihistamines. We believe barzolvolimab holds significant promise as a much needed potential treatment for patients with CIndU and look forward to presenting topline data from this study in the second half of the year.”
In February 2024, the Company presented positive 12 week primary endpoint results from its ongoing Phase 2 study of barzolvolimab in the most common form of chronic urticaria—chronic spontaneous urticaria (CSU).
PHOTO CREDIT: DermNetNZ