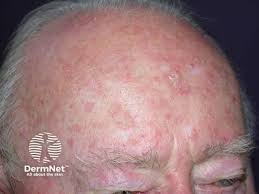
The U.S. Food and Drug Administration (FDA) has approved the use of up to three tubes of Biofrontera Inc.’s Ameluz (aminolevulinic acid HCI) topical gel, 10% in one treatment with the BF-RhodoLED or RhodoLED XL lamps.
This is up from the previous maximum of one tube. Ameluz is recognized by the FDA for both lesion-directed and field-directed treatment of actinic keratoses.
The Supplemental New Drug Application (sNDA) was supported by two Phase 1 safety studies involving 116 patients. The studies showed that that the increased dosage did not raise the blood concentration of the active ingredient to levels known to cause side effects.
The systemic and application site adverse events observed with the triple-tube dosage were comparable to those seen with one tube.
“It’s now easier to treat larger areas on patients as this was previously dose limited,” says Vishal Patel, MD, an Associate Professor of Dermatology at the GW School of Medicine & Health Sciences and the Director of the Cutaneous Oncology Program at the GW Cancer Center in Washington, DC. “This makes it more convenient for patients [and] potentially reduces frequency of treatment. It should also make it easier to incorporate photodynamic therapy (PDT) into the treatment algorithm for field cancerization/actinic keratosis, which has been shown to be one of the most tolerable and effective ways to treat pre cancers and preventive cancer development.”
PHOTO CREDIT: DermNet