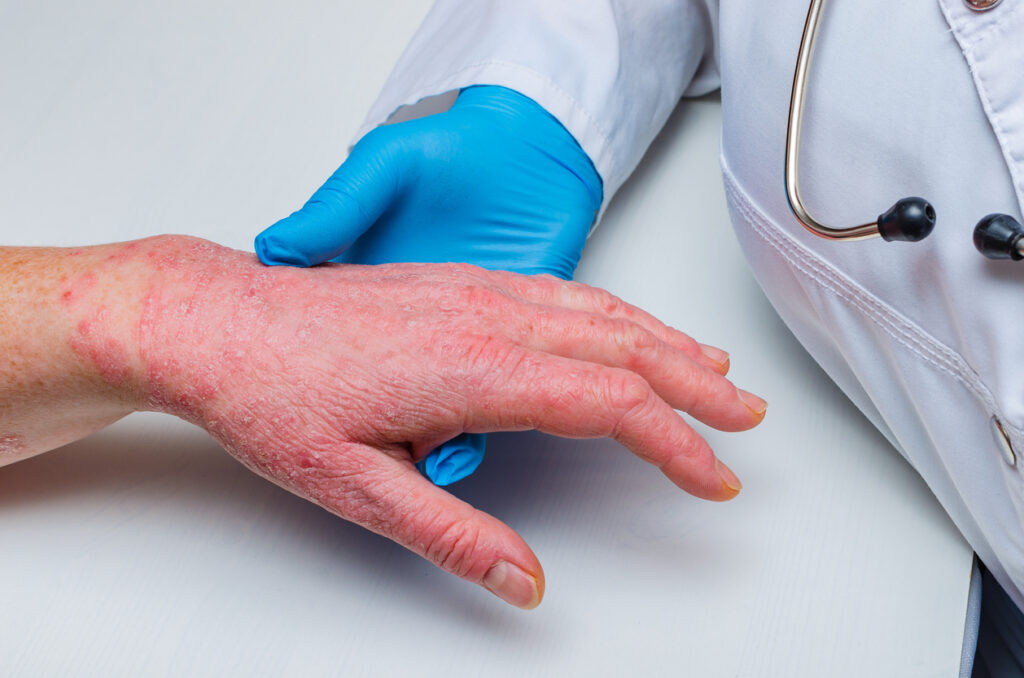
Fully 65% of dermatology providers agree there is a lack of education and understanding of chronic hand eczema (CHE) as a condition separate from atopic dermatitis (AD), according to a new survey.
The Talk to the Hand survey, which included responses from 100 doctors and 92 nurse practitioners or physician assistants in dermatology, was commissioned by LEO Pharma Inc. and conducted by Ipsos. Half of these dermatology providers (51%) said that the current treatments approved for moderate-to-severe AD are insufficient for treating moderate-to-severe CHE. In addition, almost two-thirds of survey respondents consider CHE more frustrating to manage compared to other chronic skin conditions.
“One of the challenges of chronic hand eczema is that not only is the terminology unfamiliar to a lot of dermatologists in this country, but the concept of the disease state itself also has a lack of familiarity,” says Raj Chovatiya, MD, PhD, Founder and Director of the Center for Medical Dermatology and Immunology Research in Chicago, IL. “I think that the absence of a targeted therapy for this disease state has also led to a poor understanding of what CHE is.”
There is currently no U.S. Food and Drug Administration-approved treatment for CHE.
Additional Findings
Additional survey findings include:
- Patients with CHE spend a lot of time at the doctor’s office. Sixty-two percent of surveyed dermatology providers say their moderate-to-severe CHE patients average three to four office visits per year. Twenty-seven percent report their patients come to the office five to six times a year.
- According to respondents in the survey, a lack of suitable treatment options for CHE means doctors write a lot of prescriptions but notice low medication adherence. Sixty-one percent of these dermatology providers say, on average, their moderate to severe CHE patients are currently on three to four different medications to treat CHE. However, almost a quarter (24%) estimate between 31%-50% of their CHE patients are non-compliant.
Lack of CHE Treatments
A systemic therapy, alitretinoin, is approved in Canada, the European Union, South Korea, and Israel. Dupilumab (Dupixent, Sanofi & Regeneron) was FDA-approved in January 2024 for treating moderate-to-severe AD affecting the hands and/or feet in people aged 12 and older. Unfortunately, none of these treatments are one-hand-fits-all.
This is why there is a great deal of excitement about delgocitinib cream (LEO Pharma, Anzupgo), a pan-Janus kinase (JAK)-inhibitor, which is in clinical trials in the U.S. Delgocitinib cream is currently approved in the European Union and Switzerland for treating moderate-to-severe CHE in adults for whom topical corticosteroids are inadequate or inappropriate.
Diagnosing CHE
CHE is a heterogeneous disease. “It is not AD. CHE is multifactorial and multidimensional, and we know that the disease state itself consists of a variety of morphologic and etiologic subtypes, and therein lies the challenge, but also the advantage of such a diagnosis,” he says. “Morphologically, eczema can present in the hands in many different ways, and it can be caused by a combination of irritant and allergic contact dermatitis, protein or contact dermatitis, or contact urticaria as well as atopic dermatitis,” Dr. Chovatiya explains.
The CHE diagnosis allows dermatologists to group a lot more things together, which can otherwise be difficult to disentangle. “It actually makes it very convenient.”
Diagnosing CHE starts with asking simple questions about a patient’s medical history, risk factors, and symptoms.
“Knowing about pain and itch is important in this disease state because both are common symptomatic complaints that drive a lot of the quality of life burden,” he says. “A lot of people have work, productivity, and activity impairment with CHE, so it’s really helpful to know how this disease is impacting their life.”
Asking about occupation and daily activities can also shed some light on the diagnosis. “This gives you an idea of the types of exposures that they may have on a daily basis,” he says. “All of that taken together, plus a Gestalt assessment of severity through some type of physician’s global assessment, should be enough to get you where you need in terms of making a diagnosis and gauging severity of CHE.”
Data from the second phase of the Talk to the Hand survey, which found 96% of surveyed U.S. dermatology providers agree moderate-to severe-CHE has a strong impact on patients’ work and home life, will be released in Q2 2025. Stay tuned.