By Meshi Paz, BS, and Peter A. Lio, MD
- Introduction
- Cellular Connections
- Clinical Examples in Skin Diseases
- Therapeutic Implications
- Future Developments
Introduction
The inextricable connection between the mind and the body poses a complex set of questions to medical research since it involves a variety of organs and systems. The nervous system alone communicates with nearly every part of the body. Specifically, its tie with the immune system influences the skin, and of course, the body’s hormones demonstrate the complex phenomenon referred to as the Neuro-Immuno-Cutaneous-Endocrine network, or “NICE.” With the skin being the largest organ in the body and the primary interface to the external world, the NICE network serves an essential role in a number of dermatologic diseases. Understanding the integration among the organs and systems involved in the NICE network should allow for a more comprehensive approach to both understanding and treating a variety of diseases.
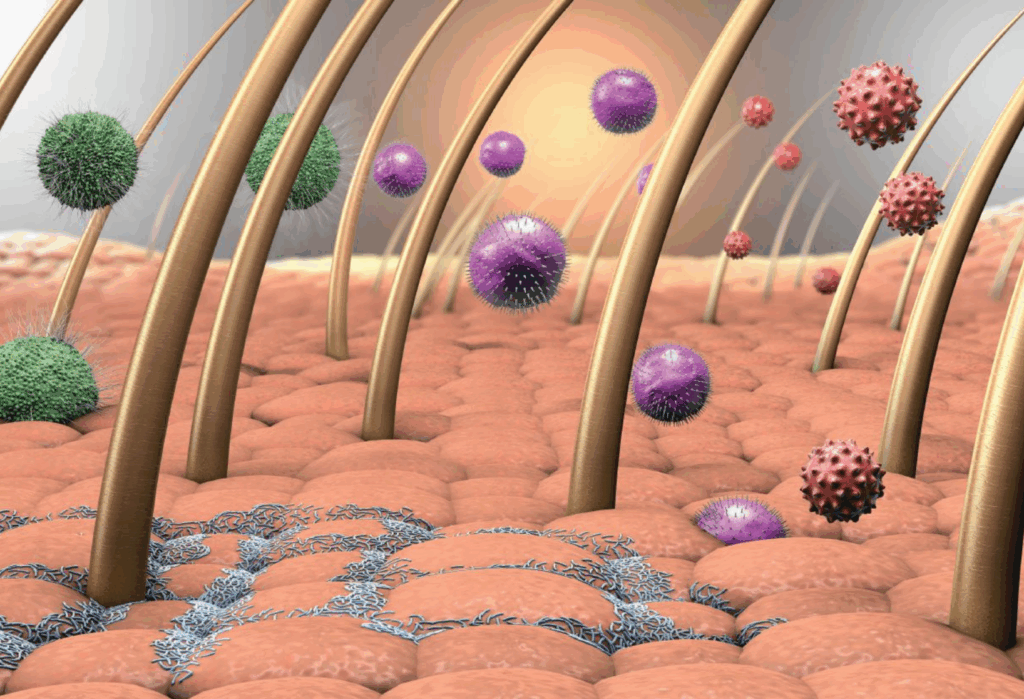
The NICE system communicates using neuropeptides, cytokines, neurotransmitters, and hormones (see Figure 1). The skin serves as a connection between the neuroendocrine and immune systems.
This link lies in the production of substance P and calcitonin gene-related peptide (CGRP) by sensory nerve endings in the epidermis. Both substances mediate many biological functions, including pain sensation, inflammation, and cell growth, thus demonstrating their integral role in the NICE network. Beyond this, there are many interconnections within the NICE system’s individual components.
Cellular Connections
The skin is an essential component of the immune system, acting as the initial barrier against external pathogens. Beyond its protective barrier function, the skin is also home to numerous immunocompetent cells, making it a pivotal element of the innate immune system. Langerhans cells within the skin serve as the antigen-presenting cells of the epidermis, stimulating the T-cell response system. Dendritic cells, keratinocytes, and melanocytes are also known to possess some immune properties, such as initiating cytokine and chemokine responses. Through these cells, the skin sends signals to evoke an immune response when stimulated by a pathogen. The skin also produces antimicrobial peptides and lipids. Antimicrobial peptides, specifically cathelicidins and defensins, fight off pathogens by disrupting bacterial membranes. Substance P and CGRP, as mentioned previously, play an essential role in proinflammatory immune functions, modulating dermal blood flow. These substances respond to stimulation by sensory nerves in the skin to induce vasodilation, increase vascular permeability, and recruit inflammatory cells. Lipids secreted by the skin also assist in its antimicrobial function. Of course, the skin’s function in wound healing is essential in its immune properties. Through hemostasis, the skin induces blood clotting around the injury to prevent further bleeding. Inflammation of the skin is known to be a response by immune cells, including neutrophils, monocytes, and lymphocytes. Proliferation and remodeling of the skin allow for restoration of the skin back to its homeostatic state.
The skin contains unmyelinated mechanosensory nerve endings, which it utilizes to send signals to the brain upon external stimulation, such as touch or irritation. Epidermal cells, such as keratinocytes, melanocytes, Langerhans cells, and Merkel cells, communicate with neurons through chemical messengers like substance P and CGRP. Keratinocytes, which contribute to the strong protective barrier of the skin, proliferate in response to neurotransmitters and neuropeptides released by the neurons. These cells serve as the basis of communication between skin cells and neurons by detecting sensory stimuli and converting them into nerve impulses.
Furthermore, keratinocytes, along with melanocytes, are thought to act as part of the cholinergic system: keratinocytes have been shown to produce acetylcholine while melanocytes possess the vesicular acetylcholine transporter. Merkel cells are often found close to sensory neurons, forming Merkel-neurite complexes. These cells have demonstrated afferent communication with these neurons through synaptic transmission, which identifies these cells as mechanosensory epidermal or “touch” cells. It has been found that small neurotransmitters called monoamines are stored and potentially produced in Merkel cells, further illustrating the neuro-cutaneous connection. Some of these monoamines include serotonin (5HT) and catecholamines. Further, vascular smooth muscle cells and endothelial cells in the dermis of the skin are associated with mechanosensory neurons as well.
The cutaneous-endocrine connection demonstrates the role of skin as an endocrine organ through its ability to generate and metabolize hormones. The functional units of this connection are hair follicles and associated sebaceous glands. Androgens, for instance, have been shown to be produced by these structures, through the conversion of dehydroepiandrosterone and androstenedione into testosterone, androstenedione, and eventually 5α-dihydrotestosterone. Androgen receptors are also present in the pilosebaceous unit as well as in keratinocytes, showing the endocrine communication within the skin. Other receptors, including those for peptide and steroid hormones, are expressed throughout many types of skin cells, indicating significant biological effects of the hormones in the skin. For example, proopiomelanocortin (POMC), a peptide hormone, acts on the skin to initiate melanogenesis when stimulated by corticotropin-releasing hormone (CRH). Both of these hormones act on skin receptors after circulating through the body and are also made endogenously within skin cells. This, once again, supports the interplay between the skin and its endocrine functions.
When studied together, the components of the NICE system possess unique interconnections that pose interesting potential for further research and mitigation. One example of this is the local corticotropin-proopiomelanocortin (CRH-POMC) axis—a key pathway involved in glucocorticoidogenesis. Similar to that of the hypothalamic-pituitary-adrenal axis of the central nervous system, this cutaneous CRH/POMC axis serves as the local cutaneous equivalent that responds to stressors. Since the skin is located at the barrier between the external and internal environments, it is the first part of the body to receive stressful environmental stimuli, such as ultraviolet radiation, injury, toxins, temperature changes, and infection. In turn, the skin responds quickly by generating cytokines and CRH from dermal cells and nerves. CRH stimulates POMC, which works with the cytokines to act back on skin cells by autocrine and paracrine signaling, generating a local response. Although further research needs to be done to understand the exact local response, it is thought that the CRH-POMC axis is involved in inflammation and cell proliferation. Although further research needs to be conducted, it is thought that substance P, CGRP, glucocorticoids, and other neuropeptides work in conjunction with the endocrine functions of the skin to further amplify the proliferation of skin cells. This cooperation between the various organ systems emphasizes the intertwined nature of the NICE network (see Figure 2).
Many dermatological diseases, specifically those characterized as involving barrier dysfunction, demonstrate the functional and dynamic relationship of the NICE system. Its role in cutaneous diseases is driven by the hypothalamic-pituitary-adrenal (HPA) axis, which induces a hormone response to psychological stress with the end result being the regulation of cortisol production. In essence, the rise in cortisol from the activation of this neuroendocrine system serves an immunosuppressive effect. However, chronic stress and the associated chronic increase in cortisol lead to a tolerance to these anti-inflammatory effects and thus an altered immune system. Psoriasis, acne, and eczema are just some examples of dermatological diseases that display this NICE connection.
Clinical Examples in Skin Diseases
Psoriasis serves as an excellent showcase. The mechanism of this disease seems to involve an inflammatory process driven by cytokines and neuroendocrine communicators. Specifically, both innate immune cells—dendritic cells and neutrophils— as well as adaptive immune system cells—CD4+ and CD8+ T lymphocytes—communicate with keratinocytes and melanocytes to mediate the expression of psoriasis. These skin cells demonstrate their immune function through their proinflammatory cytokine signaling, including interferon (IFN)-γ, timot nrecrosus facypr (TNF)-α, and interleukin (IL)-17. High substance P and CGRP levels have been correlated with psoriasis. These neuropeptides are thought to contribute to the hyperproliferation seen in this disease. It has even been found that denervation alleviates symptoms of psoriasis, indicating that neuropeptides may be a source of the clinical presentation of psoriasis. Further, the neuroendocrine basis of psoriasis involves the neurotransmitter serotonin (5 hydroxytryptamine; 5-HT). Serotonin levels are known to be reduced in psychological stress. This decrease in serotonin is thought to stimulate the proinflammatory cytokines listed previously. With this, the basis of psoriasis and stress-induced flare-ups is noted through the relationship between inflammation and serotonin.
Along similar lines, atopic dermatitis (AD) exhibits its interplay with the NICE system. Although the exact mechanism remains unclear, it is thought that the biological response of AD flares may be stimulated by an environmental trigger in genetically predisposed individuals. Stress, both psychological and physiological, is thought to be an important environmental trigger of AD, triggering an HPA dysregulation as well as a hyperactive T-cell response. High levels of activated T cells in patients with AD have been noted in the skin as well as in the systemic circulation. Particularly, Th2 response predominates in AD, which indicates an antibody-mediated immune response. Neuropeptides, including substance P and CGRP, are released from local cutaneous nerves and elicit vasodilation, which contributes to the redness of the skin in AD. These neuropeptides also recruit mast cells and other immune cells to the site of inflammation. Mast cells degranulate and release histamine, which serves as the basis of the AD rash formation. Itch perception seen in AD is also thought to have similar neural pathways as those relating to pain. Furthermore, it has been found that glucocorticoids are key mediators in the detrimental impacts of stress on the skin. Although the mechanism of this is still up for debate, it has been proven that glucocorticoids play an essential role in barrier dysfunction. In response to psychological stress, glucocorticoid production increases, eliciting a disruption in barrier homeostasis. This suggests a link that may explain the exacerbation of diseases like AD.
Acne is another common skin disorder thought to be associated with the NICE network. Acne pathogenesis lies mainly in the dysfunction and inflammation of the pilosebaceous unit due primarily to hypersecretion of sebum, alterations in the fatty acid composition of the sebum, or hyperkeratinization leading to obstruction of the follicles. Acne is also known to be caused by dysregulated hormones, neurotransmitters, and innate and adaptive immune systems. For example, the hormone insulin-like growth factor-1 (IGF-1) has been shown to be elevated in patients with acne. Other hormones, including testosterone and progesterone, have been associated with the presence of acne. Further, androgen receptors located on the sebaceous gland indicate that testosterone and other androgens interact with the gland. The sebaceous gland is also known to synthesize testosterone de novo from cholesterol. Psychological stress is also known to exacerbate acne. This stress is modulated through the HPA axis, demonstrating the link to the neuroendocrine system. The skin of individuals with acne has been found to present with an increase in mast cells and nerves containing substance P. Substance P serves a role in the inflammation of a pilosebaceous unit, thus indicating its potential function in the pathogenesis of acne. In turn, the pilosebaceous unit proliferates and increases lipid synthesis. Substance P, along with CGRP, initiates the inflammatory process by stimulating the release of proinflammatory cytokines and by initiating the proliferation and degranulation of mast cells. As such, chronic stress generates an increase in androgen and cytokine secretion, leading to an increase in the activity of the pilosebaceous unit. The cooperation of the NICE network thus contributes to the progression of acne.
Therapeutic Implications
The NICE system has also been a proven target of therapeutic approaches. By understanding this network, clinicians can treat patients more holistically by considering the interconnectedness of various body systems and their role in overall health. From this perspective, cutaneous inflammation can be a potential indicator of an underlying inflammatory disorder, hormonal dysfunction, or psychological stress. This may encourage the exploration of deeper problems, hopefully allowing for more comprehensive treatment. Clinicians are also able to deliver more contextualized care by recognizing the impact of external influences on a current problem. Many studies have already proven the implication of using the crosstalk of the NICE system as a treatment method. For one, psychological interventions, including stress reduction and hypnosis, have been used to target cutaneous and immunologic inflammation. Chemical mediators of the NICE system, including cytokines and hormones, present opportunities for further research on the therapeutic implications of this network.
Future Developments
The NICE network demonstrates the complex and fascinating interconnection of the mind, body, and external world through the skin. The integration among the nervous, immune, endocrine, and cutaneous systems highlights the importance of considering the crosstalk of these systems in the context of dermatologic diseases. While the NICE system has been a proven target of therapeutic approaches, there is still much to learn about its components and their interplay. The skin serves as a critical connection between the various systems, playing an essential role in potential therapeutic interventions. Further research into the NICE network could lead to the development of more effective treatments for dermatologic diseases as well as more holistic patient care. Therefore, future developments should focus on advancing our knowledge of the NICE system and its potential for clinical applications.
FOR FURTHER READING
- Arck PC, Slominski A, Theoharides TC, Peters EMJ, Paus R. Neuroimmunology of stress: Skin takes center stage. J Invest Dermatol. 2006;126(8):1697–1704. https://pubmed.ncbi.nlm.nih.gov/16845409/
- Bakry OA, El Shazly RMA, El Farargy SM, Kotb D. Role of hormones and blood lipids in the pathogenesis of acne vulgaris in non-obese, non-hirsute females. Indian Dermatol Online J. 2014;5(Suppl 1):S9–S16. https://pubmed.ncbi.nlm.nih.gov/25506579/
- Bellavance M, Rivest S. The HPA – immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front Immunol. 2014:5:136. https://pubmed.ncbi.nlm.nih.gov/24744759/
- Brazzini B, Ghersetich I, Hercogova J, Lotti T. The neuro-immuno-cutaneous-endocrine network: Relationship between mind and skin. Dermatol Ther. 2003;16(2):123–131. https://pubmed.ncbi.nlm.nih.gov/12919114/
- Chen W-C, Zouboulis CC. Hormones and the pilosebaceous unit. Dermatoendocrinol. 2009;1(2):81–86. https://pubmed.ncbi.nlm.nih.gov/20224689/
- Elwary SMA, Chavan B, Schallreuter KU. The vesicular acetylcholine transporter is present in melanocytes and keratinocytes in the human epidermis. J Invest Dermatol. 2006;126(8):1879–1884. https://pubmed.ncbi.nlm.nih.gov/16763548/
- Ganceviciene R, Böhm M, Fimmel S, Zouboulis CC. The role of neuropeptides in the multifactorial pathogenesis of acne vulgaris. Dermatoendocrinol. 2009;1(3):170–176. https://pubmed.ncbi.nlm.nih.gov/20436885/
- Garg A, Chren MM, Sands LP, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: Implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137(1):53–59. https://pubmed.ncbi.nlm.nih.gov/11176661/
- Gaspar NK, Aidé MK. Atopic dermatitis: allergic dermatitis or neuroimmune dermatitis? An Bras Dermatol. 2016;91(4):479–488. https://pubmed.ncbi.nlm.nih.gov/27579744/
- Hoffman BU, Baba Y, Griffith TN, et al. Merkel cells activate sensory neural pathways through adrenergic synapses. Neuron. 2018;100(6):1401-1413.e6. https://pubmed.ncbi.nlm.nih.gov/30415995/
- Hutter V, Kirton SB, Chau DYS. 15 – immunocompetent human in vitro skin models. In: Marques AP, Pirrao RP, Cerqueira MT, Reis RR, eds. Skin Tissue Models. Boston, MA: Academic Press. 2018:353–373.
- Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019;40(2):84–92. https://pubmed.ncbi.nlm.nih.gov/30819278/
- Kim YJ, Granstein RD. Roles of calcitonin gene-related peptide in the skin, and other physiological and pathophysiological functions. Brain Behav Immun Health. 2021:18:100361. https://pubmed.ncbi.nlm.nih.gov/34746878/
- Lonnne-Rahm SB, Rickberg H, El-Nour H, Mårin P, Azmitia EC, Nordlind K. Neuroimmune mechanisms in patients with atopic dermatitis during chronic stress. J Eur Acad Dermatol Venereol. 2008;22(1):11–18. https://pubmed.ncbi.nlm.nih.gov/18181968/
- Martins AM, Ascenso A, Ribeiro HM, Marto J. The brain-skin connection and the pathogenesis of psoriasis: A review with a focus on the serotonergic system. Cells. 2020;9(4):796. https://pubmed.ncbi.nlm.nih.gov/32224981/
- Mashaghi A, Marmalidou A, Tehrani M, Grace PM, Pothoulakis C, Dana R. Neuropeptide substance P and the immune response. Cell Mol Life Sci. 2016;73(22):4249–4264. https://pubmed.ncbi.nlm.nih.gov/27314883/
- Morey JN, Boggero IA, Scott AB, Segerstrom SC. Current directions in stress and human immune function. Curr Opin Psychol. 2015:5:13–17. https://pubmed.ncbi.nlm.nih.gov/26086030/
- Nguyen AV, Soulika AM. The dynamics of the skin’s immune system. Int J Mol Sci. 2019;20(8):1811. https://pubmed.ncbi.nlm.nih.gov/31013709/
- O’Sullivan RL, Lipper G, Lerner EA. The neuro-immuno-cutaneous-endocrine network: Relationship of mind and skin. Arch Dermatol. 1998;134(11):1431–1435. https://pubmed.ncbi.nlm.nih.gov/9828880/
- Rassouli O, Liapakis G, Venihaki M. Role of central and peripheral CRH in skin. Curr Mol Pharmacol. 2018;11(1):72–80. https://pubmed.ncbi.nlm.nih.gov/27784217/
- Salmon JK, Armstrong CA, Ansel JC. The skin as an immune organ. West J Med. 1994;160(2):146–152. https://pubmed.ncbi.nlm.nih.gov/8160465/
- Suárez AL, Feramisco JD, Koo J, Steinhoff M. Psychoneuroimmunology of psychological stress and atopic dermatitis: Pathophysiologic and therapeutic updates. Acta Derm Venereol. 2012;92(1):7–15. https://pubmed.ncbi.nlm.nih.gov/22101513/
- Vidal Yucha SE, Tamamoto KA, Kaplan DL. The importance of the neuro-immuno-cutaneous system on human skin equivalent design. Cell Prolif. 2019;52(6):e12677. https://pubmed.ncbi.nlm.nih.gov/31441145/
- Wardhana M, Windari M, Puspasari N, Suryawati N. Role of serotonin and dopamine in psoriasis: A case-control study. Open Access Maced J Med Sci. 2019;7(7):1138–1142. https://pubmed.ncbi.nlm.nih.gov/31049096/
- Xu X, Yu C, Xu L, Xu J. Emerging roles of keratinocytes in nociceptive transduction and regulation. Front Mol Neurosci. 2022:15:982202. https://pubmed.ncbi.nlm.nih.gov/36157074/
- Zouboulis CC. The human skin as a hormone target and an endocrine gland. Hormones (Athens). 2004;3(1):9–26. https://pubmed.ncbi.nlm.nih.gov/16982574/
- Zouboulis CC. The skin as an endocrine organ. Dermatoendocrinol. 2009;1(5):250–252. https://pubmed.ncbi.nlm.nih.gov/20808511/
- Zouboulis CC. Endocrinology and immunology of acne: Two sides of the same coin. Exp Dermatol. 2020;29(9):840–859. https://pubmed.ncbi.nlm.nih.gov/32779248/
ABOUT THE AUTHORS
Meshi PAZ, BS, is a third-year medical student at Tulane University School of Medicine in New Orleans, LA.
Peter A. Lio, MD, is a Clinical Assistant Professor of Dermatology and Pediatrics at Northwestern University Feinberg School of Medicine and a partner at Medical Dermatology Associates of Chicago in Chicago, IL.
DISCLOSURES
Meshi Paz, BS, reports no disclosures.
Peter A. Lio, MD, reports being on the speaker’s bureau for AbbVie, Arcutis, Eli Lilly, Galderma, Hyphens Pharma, Incyte, La Roche-Posay/L’Oreal, Pfizer, Pierre-Fabre Dermatologie, Regeneron/Sanofi Genzyme, and Verrica. He reports consulting/advisory boards for Alphyn Biologics, AbbVie, Almirall, Amyris, Arcutis, ASLAN, Astria Therapeutics, Boston Skin Science, Bristol-Myers Squibb, Burt’s Bees, Castle Biosciences, Codex Labs, Concerto Biosci, Organon (Dermavant), Eli Lilly, Galderma, LEO Pharma, Lipidor, L’Oreal, Merck, Micreos, MyOR Diagnostics, Pelthos Therapeutics, Regeneron/Sanofi Genzyme, Sibel Health, Skinfix, Soteri Skin, Stratum Biosciences, Sun Pharma, Theraplex, Thimble Health, UCB, Unilever, Verdant Scientific, Verrica, and Yobee Care. Dr. Lio has stock options with Alphyn Labs, Codex Labs, Concerto Biosci, Soteri Skin, Stratum Biosciences, Thimble, Yobee Care, and Verdant Scientific. In addition, he has a patent pending for a Theraplex product with royalties paid.