With Jack D. Sobel, MD

Dr. Jack Sobel talks about ibrexafungerp, a first-in-class oral triterpenoid antifungal agent approved for the treatment of acute vulvovaginal candidiasis.
“Ibrexafungerp (Brexafemme, Scynexis) is a first-in-class oral triterpenoid antifungal agent approved for the treatment of acute vulvovaginal candidiasis (VVC). Post-approval clinical experience is needed to fully understand the optimal use of ibrexafungerp as it is with any novel drug. Nonetheless, the availability of ibrexafungerp is an exciting development for expanding the therapeutic options for patients with VVC,” said Jack D. Sobel, MD.
Dr. Sobel, Professor of Medicine (Infectious Diseases) and Obstetrics and Gynecology, Wayne State University School of Medicine, Detroit, Michigan, was an investigator in premarketing clinical studies that supported FDA approval of ibrexafungerp for treating acute VVC. Although women with symptoms of VVC most often seek care from their gynecologist or primary care physicians, information about ibrexafungerp is of interest to dermatologists who may be called on to treat VVC because of its association with antibiotics used to treat acne, rosacea, and other dermatologic diseases.

“Single-dose oral fluconazole is a widely used option for treating VVC, and a good choice considering its overall efficacy, safety, and availability in generic form. Nevertheless, fluconazole suffers from a variety of limitations, including its potential for cytochrome P450 (CYP)-mediated drug interactions and for causing adverse events that include alopecia and idiosyncratic hepatotoxicity. Moreover, Candida resistance to fluconazole is a growing concern,” said Dr. Sobel.
“Ibrexafungerp has a different mechanism of action compared to the azole antifungals. It is a glucan synthase enzyme inhibitor that blocks the production of (1,3)-β-D-glucan polymers that are integral for fungal cell wall strength, and it has broad-spectrum anti-Candida fungicidal activity, including against C. albicans and other Candida isolates showing echinocandin and azole resistance. Ibrexafungerp was well-tolerated in clinical trials without evidence of causing renal, hepatic, or systemic safety issues, and drug interaction studies indicate that ibrexafungerp has a low potential for any cytochrome P450-mediated drug interactions.”
Phase 3 study data
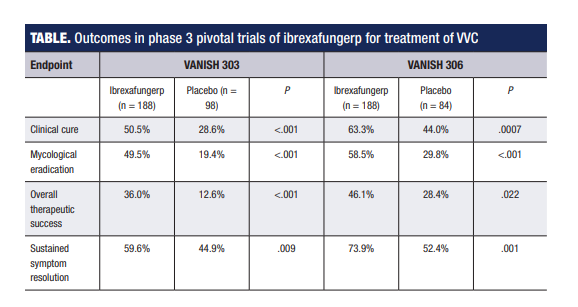
The pivotal trial program investigating ibrexafungerp involved two identically designed studies – VANISH 303, which was conducted entirely in the United States, and VANISH 306, which had sites in the United States and Bulgaria.1,2
Both studies randomized participants 2:1 to a 1-day course of ibrexafungerp 300 mg (2x 150 mg) BID or placebo BID. Both studies met their primary end point that assessed the percentage of patients achieving clinical cure, which was stringently defined according to current FDA criteria as complete resolution of VVC signs and symptoms (Table). Ibrexafungerp also demonstrated statistical superiority to placebo in secondary end points analyzing mycological eradication and overall success rates (absence of signs and symptoms plus vaginal swab culture negative for Candida species growth) (Table).
Post-hoc analyses in VANISH 303 showed outcomes with ibrexafungerp were similar in Black women and those with higher body mass index (>35 kg/m2) compared with the overall population.
Ibrexafungerp was generally well-tolerated and had a favorable safety profile across the premarketing clinical trial program, including in phase 1 studies where subjects received single oral doses of up to 1600 mg and multiple oral doses of up to 800 for 28 days. The most common treatment-emergent adverse events associated with ibrexafungerp in the phase 3 studies and occurring at rates higher than in the placebo group were gastrointestinal in nature and included diarrhea/loose stool (16.7%), nausea (11.9%), and abdominal pain (11.4%). These adverse events were generally mild to moderate in severity and did not lead to treatment discontinuation.
Clinical perspectives
Available data indicate that compared with oral fluconazole, ibrexafungerp is at least as effective as a single dose of fluconazole for treating uncomplicated symptomatic VVC and may offer better efficacy based on analyses of sustained response rates. While medication cost/insurance coverage is usually the deciding factor in treatment selection, certain patient characteristics might favor ibrexafungerp as the antifungal drug of choice for certain women with VVC, according to Dr Sobel.
He said, “Ibrexafungerp should be considered for women with fluconazole allergies or a history of intolerance, and it is likely preferred for patients with refractory symptomatic disease as they may be expected to have infection with a fluconazole-resistant species.”
Dr. Sobel noted that like fluconazole, ibrexafungerp is contraindicated for use in pregnancy due to the risk of fetal harm.
“Therefore, we still await an alternative oral treatment for VVC that fills the gap for use during pregnancy,” he said.
He also observed that women enrolled in the ibrexafungerp clinical trials predominantly had mild to moderately severe uncomplicated VVC caused by C. albicans.
“A phase 3 study is investigating ibrexafungerp for the treatment of recurrent VVC. Research is also needed to determine its efficacy for treating severe forms of VVC and cases caused by non-albicans Candida sp. or azole-resistant Candida. Fortunately, the latter affect only a minority of women with VVC.
By Cheryl Guttman Krader
________________________________________________________________________________________________________________
References
1. Schwebke JR, Sobel R, Gersten JK, et al. Ibrexafungerp versus placebo for vulvovaginal candidiasis treatment: a phase 3, randomized, controlled superiority trial (VANISH 303). Clin Infect Dis. 2021 Sep 1:ciab750. doi: 10.1093/cid/ciab750. Epub ahead of print.
2. Sobel R, Nyirjesy P, Ghannoum MA, et al. Efficacy and safety of oral ibrexafungerp for the treatment of acute vulvovaginal candidiasis: a global phase 3, randomised, placebo-controlled superiority study (VANISH 306). BJOG. 2022;129(3):412-420. doi: 10.1111/1471-0528.16972.
________________________________________________________________________________________________________________
Disclosures
Dr. Sobel is a consultant to Scynexis.