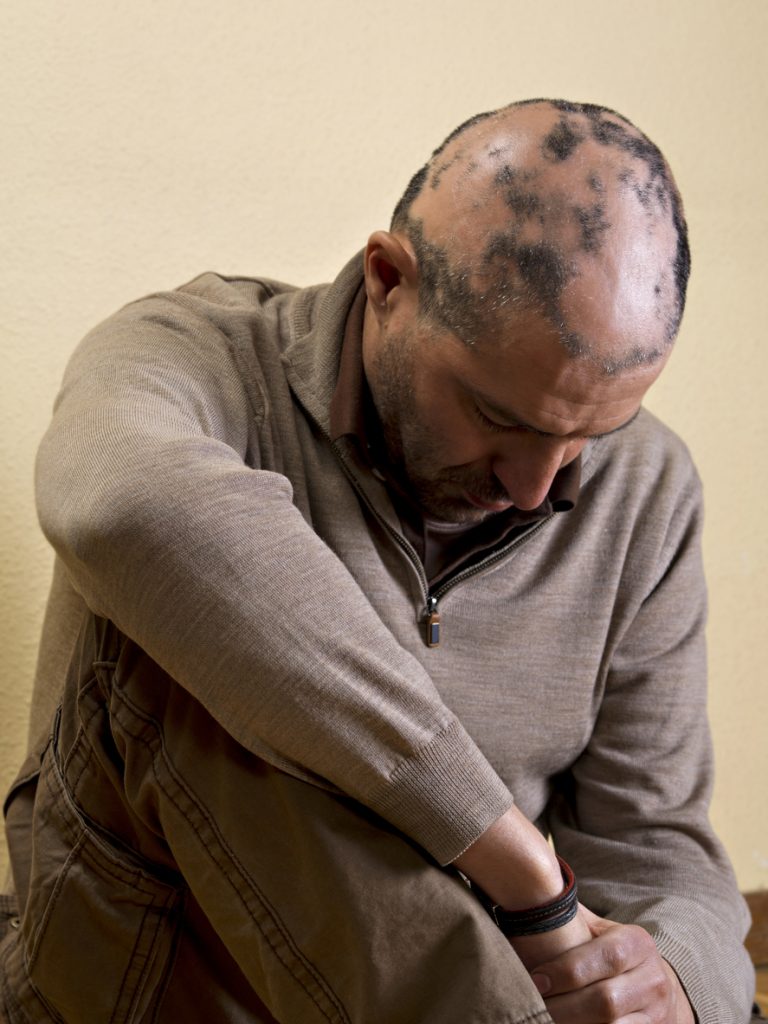
Twice-daily dosing of deuruxolitinib resulted in significant hair regrowth for adults with moderate-to-severe alopecia areata (AA), and these improvements were visible as early as 12 weeks and lasted throughout the 24-week study, according to the Phase 3 THRIVE-AA2 trial.
The new results, presented as a late breaker at Maui Derm 2024, are consistent with earlier data on the Janus kinase (JAK) 1/2 inhibitor, which is under development by Sun Pharma.
In the study, 517 patients aged 18 to 65 years of age with AA, ≥50% scalp hair loss (per Severity of Alopecia Tool [SALT] score), and current AA episode lasting ≥6 months to ≤10 were randomized 2:1:1 to deuruxolitinib 8 mg, deuruxolitinib 12 mg, or placebo twice daily for 24 weeks.
The primary endpoint was proportion of patients achieving absolute SALT scores of ≤20 at Week 24. Secondary endpoints assessed percentage of patients achieving absolute SALT scores of ≤10 and relative changes in SALT score over time.
Both doses of deuruxolitinib met the primary efficacy endpoint: 33.0% and 38.3% of patients treated with deuruxolitinib 8 mg and 12 mg, respectively, vs 0.8% of placebo-treated patients achieved absolute SALT scores of ≤20 at Week 24, with significant differences for both doses vs placebo as early as 12 weeks, the study showed.. Of the 8 mg and 12 mg groups, 24.9% and 26.7%, respectively, achieved SALT scores of ≤10 at Week 24 vs 0% for placebo. Relative changes from baseline and achievement of 75% and 90% improvement from baseline in SALT scores were also significant for both doses compared with placebo at Week 24, and as early as Week 4 for relative change from baseline.