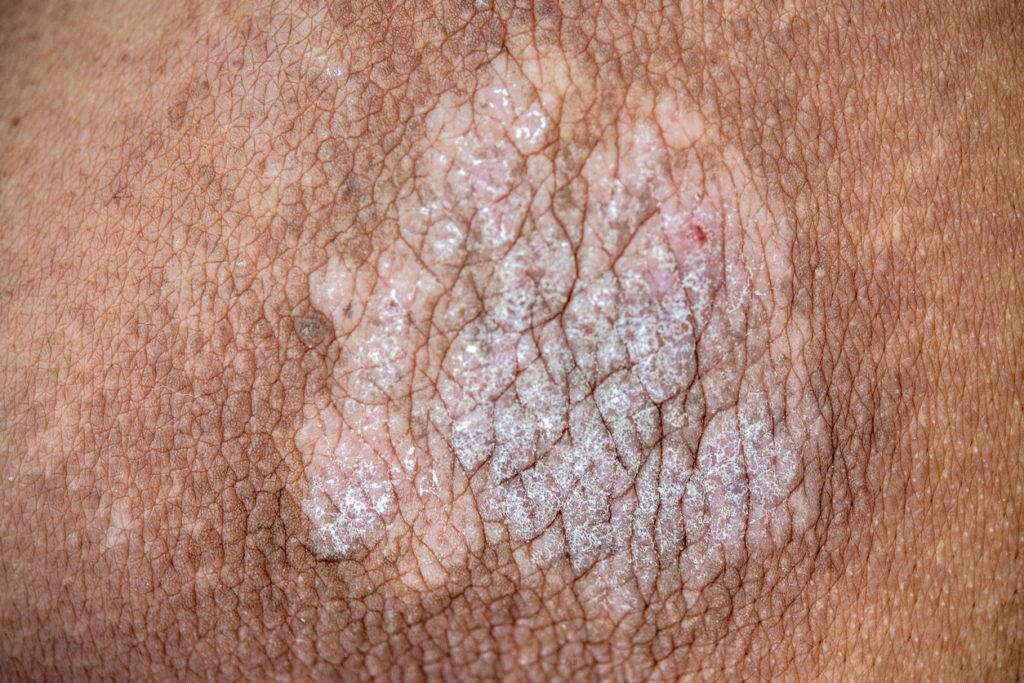
JNJ-2113, Johnson & Johnson’s oral IL-23 receptor blocker, showed positive results in moderate-to-severe plaque psoriasis, according to the Phase 2b FRONTIER 1 study published in the New England Journal of Medicine.
JNJ-2113 is the first and only investigational targeted oral peptide inhibitor designed to block the IL-23 receptor. IL-23 plays a critical role in pathogenic T-cell activation in moderate-to-severe plaque psoriasis (PsO) and underpins the inflammatory response in PsO and other dermatological and gastroenterological IL-23-mediated diseases.
For the study, 255 participants were randomized into six treatment groups (placebo [n=43], 25 mg daily [n=43], 25 mg twice-daily [n=41], 50 mg daily [n=43], 100 mg daily [n=43], and 100 mg twice-daily [n=42]). The total duration of the trial was up to 24 weeks, which included a four-week screening period, a 16-week treatment period and a four-week safety follow-up period.
The primary endpoint of the study was a reduction from baseline of at least 75% in the Psoriasis Area and Severity Index score (PASI 75 response) at Week 16. Study results demonstrated a significant dose-response on PASI 75 at Week 16 for adult patients who received JNJ-2113 compared to patients treated with placebo, with 79% of patients achieving a PASI 75 response in the highest dose group tested of 100 mg twice-daily.
The data were consistent with the secondary endpoints, with patients who received the highest dose of JNJ-2113 achieving PASI 100 of 40.5% and IGA 0 (clear skin) of 45.2%.
Improvements were also consistent across Patient Reported Outcomes through Week 16. Patients who were treated with JNJ-2113 demonstrated greater improvements from baseline in the severity of their disease-related symptoms by Week 16, as assessed by the Psoriasis Symptoms and Signs Diary (PSSD). Among patients with baseline Dermatology Life Quality Index (DLQI) scores greater than 1, significantly higher proportions of JNJ-2113-treated patients compared with placebo-treated patients achieved DLQI scores of 0/1 (no impact on quality of life) at Week 16.
In this phase 2 study, rates of adverse events (AEs) were generally similar between the JNJ-2113 and placebo groups. The most common (≥5 percent of any treatment group) AEs were COVID-19 infection, nasopharyngitis, upper respiratory tract infection, diarrhea, headache, and cough. No relationship between the JNJ-2113 dose group and the occurrence of AEs or serious AEs was observed.
Treatment with JNJ-2113 was also associated with lower serum levels of human beta-defensin 2 (hBD-2), relative to placebo, as early as Week 4, the study showed. The lowest hBD-2 level was observed with the 100 mg twice-daily dose, beginning by Week 8.1 Lower levels of hBD-2 were observed with clinical response and indicate inhibition of the IL-17/IL-23 axis.
Study author Robert Bissonnette, MD, Chief Executive Officer and Medical Director at Innovaderm Research in Montreal, Canada, chatted with TDD about the new findings.
TDD: What is the main takeaway of the from the Phase 2b FRONTIER 1 trial results published in NEJM?
Dr. Bissonnette: “JNJ-2113, a selective oral peptide targeting the IL-23 receptor, has shown biologic-like efficacy in patients with moderate to severe psoriasis. It was also well tolerated, but conclusions on safety are limited due to the small sample size of the study.”
TDD: If approved, what void may investigational JNJ-2113 fill in psoriasis?
Dr. Bissonnette: “JNJ-2113, if eventually approved, would offer patients and physicians the possibility of opting for a selective oral treatment for psoriasis. Several biologics targeting a single cytokine or receptor are approved for psoriasis, but they all require sub-cutaneous or intra-venous injections. Oral drugs are also approved, but they have an effect on multiple cytokines and their efficacy is usually lower than biologics.
TDD: What are the next steps?
Dr. Bissonnette: “Results from this study must be confirmed in phase 3. A large phase 3 program has recently been initiated with JNJ-2113 including two pivotal placebo-controlled studies, an active comparator study with deucravacitinib and a special area study where JNJ-2113 will be studied in patients with palmoplantar, genital and scalp psoriasis.“
Looking Ahead
The pivotal Phase 3 ICONIC clinical development program of JNJ-2113 in adult and adolescent patients with moderate-to-severe plaque PsO was initiated with two studies in Q4 2023 – ICONIC-LEAD and ICONIC-TOTAL – pursuant to the license and collaboration agreement between Protagonist Therapeutics, Inc. and Janssen Biotech, Inc.
ICONIC-LEAD is a randomized controlled trial (RCT) to evaluate the safety and efficacy of JNJ-2113 compared with placebo in participants with moderate-to-severe plaque PsO, with the higher efficacy bar of PASI 90 and IGA score of 0 or 1 with at least a 2-grade improvement as co-primary endpoints.
ICONIC-TOTAL is a RCT to evaluate the efficacy and safety of JNJ-2113 compared with placebo for the treatment of PsO in participants with at least moderate severity affecting special areas (e.g., scalp, genital, and/or palms of the hands and the soles of the feet) with overall IGA score of 0 or 1 with at least a 2-grade improvement as the primary endpoint. Other Phase 3 studies in the development program are expected to begin in Q1 2024, including ICONIC-ADVANCE 1 and ICONIC-ADVANCE 2, which will evaluate the safety and efficacy of JNJ-2113 compared with both placebo and deucravacitinib.
The findings from the FRONTIER 1 clinical trial suggest the potential of JNJ-2113 across the spectrum of additional IL-23-mediated diseases. The Company has initiated the Phase 2b ANTHEM-UC study to evaluate the safety and effectiveness of JNJ-2113 compared with placebo in participants with moderately to severely active ulcerative colitis.