By Denise Mann, MS
Shared-decision making focused on weighing risks versus benefits is key when choosing the most appropriate systemic atopic dermatitis (AD) treatment for a patient, according to new research presented at the 2024 American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting in Washington, DC.
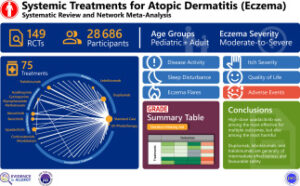
For the study, researchers reviewed the benefits and harms of all systemic and phototherapy treatments for atopic dermatitis from MEDLINE, EMBASE, CENTRAL. They performed Bayesian random-effects network meta-analyses on AD severity, itch severity, sleep disturbance, quality of life, exacerbations and adverse events. Thresholds for patient-important benefits and harms were pre-determined with a multidisciplinary panel that included patients.
In total, researchers analyzed 154 trials comprising 29,831 patients, both children and adults, and evaluated 78 interventions over a median of 13 weeks. The analysis found that high-dose upadacitinib (Rinvoq, AbbVie) was among the most effective treatments in improving multiple patient outcomes, but it was also among the most harmful in increasing any adverse event. High-dose abrocitinib (Cibinqo, Pfiizer) and low-dose upadacitinib provided intermediate effectiveness but were also among the most harmful. Dupilumab (Dupixent, Sanofi, and Regeneron Pharmaceuticals), Lebrikizumab (Ebglyss, Almirall), and tralokinumab-ldrm (Adbry, Leo) were generally of intermediate effectiveness and among the safest but modestly increased the frequency of conjunctivitis.
Low-dose baricitinib (Olumiant, Eli Lilly), was among the least effective across all AD outcomes. The benefits and harms of azathioprine, oral corticosteroids, cyclosporine, methotrexate, mycophenolate, phototherapy and many novel agents were less certain, the study showed.
“Healthcare providers and patients should engage in shared decision-making that considers the values and preferences of individual patients, the availability and costs of treatment options, in addition to the clinical evidence of benefits and harms,” says study author Derek Chu, MD, PhD, FRCPC, an Assistant Professor in the Division of Clinical Immunology & Allergy and the Director of Evidence in Allergy Research Group in the Department of Medicine and the Department of Health Research Methods, Evidence, and Impact at The Research Institute of St. Joe’s Hamilton of McMaster University in Hamilton, Ontario, Canada. “We collaboratively decide with our patients on the best treatment option based on the known benefits and harms of treatment options, our patients’ values and preferences, their clinical picture, and contextual factors. Individuals that place a very high value on improving symptoms and quality of life and lower value on avoiding uncertain harms may choose some of the most effective interventions (e.g., some of the JAK inhibitors). Individuals more concerned about avoiding serious harms and less focused on maximizing symptomatic relief are likely to choose safer and moderately less effective interventions (e.g., some of the biologics). Decisions may also be influenced by costs of medications, preference between oral and injection medications, or the frequency of taking the medication. “


