Understanding the NeuroImmuno-Cutaneous Endocrine Network and Its Role in Psoriasis, Eczema, and Acne
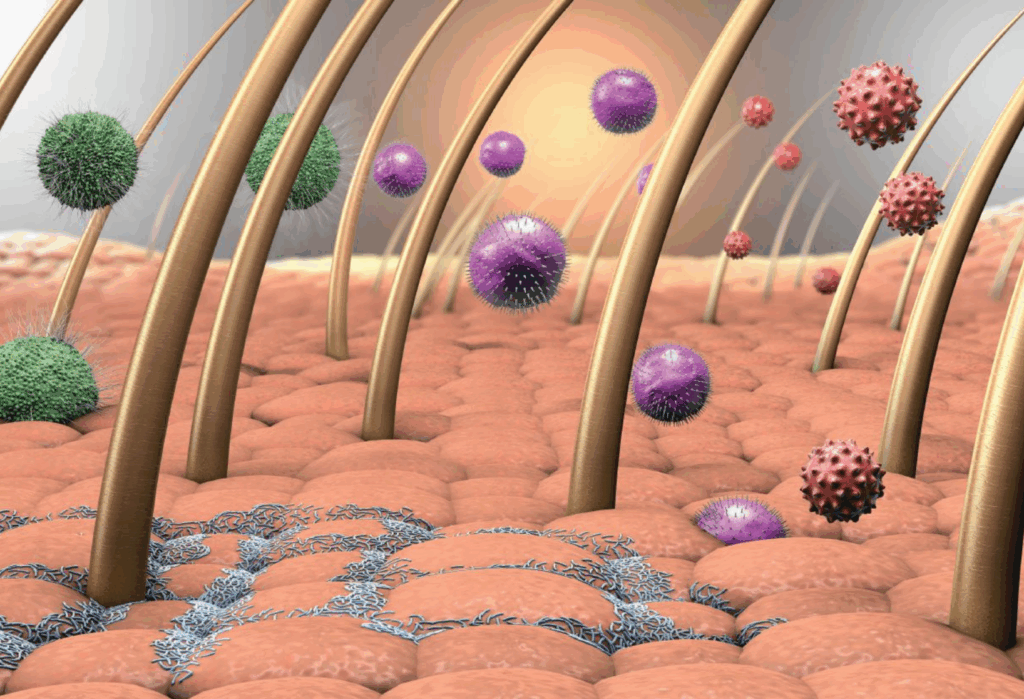
By Meshi Paz, BS, and Peter A. Lio, MD Introduction Cellular Connections Clinical Examples in Skin Diseases Therapeutic Implications Future Developments Introduction The inextricable connection between the mind and the body poses a complex set of questions to medical research since it involves a variety of organs and systems. The nervous system alone […]
