Big News: U.S. FDA Approves ZEVASKYN, the First and Only FDA-approved Product for Recessive Dystrophic Epidermolysis Bullosa
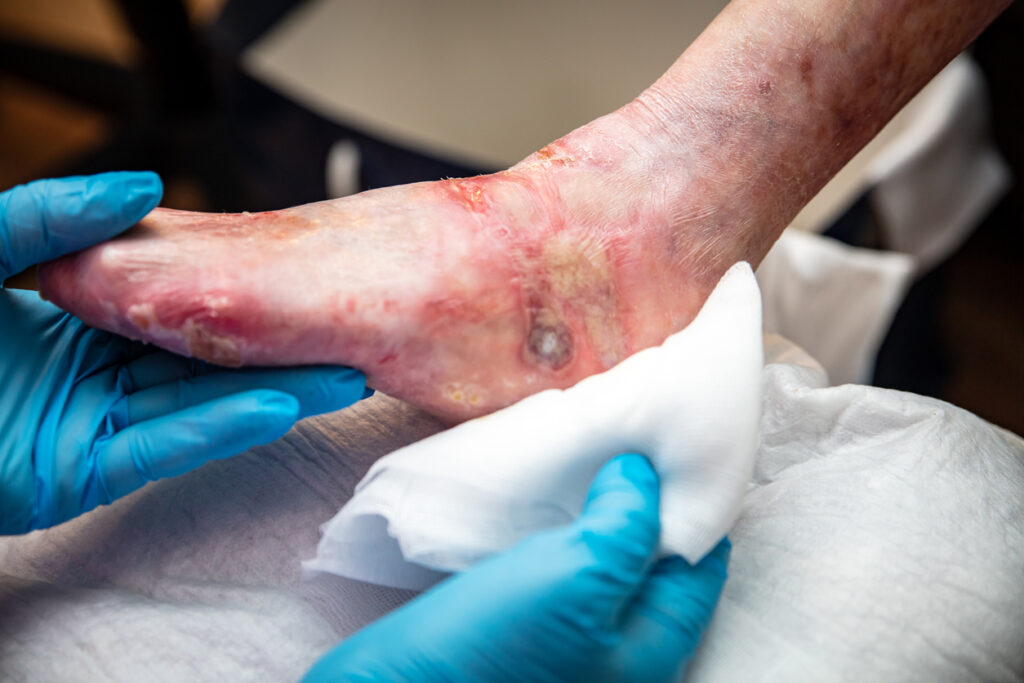
The U.S. Food and Drug Administration (FDA) has approved Abeona Therapeutics Inc.’s ZEVASKYN (pronounced as ‘ZEE-vah-skin’) (prademagene zamikeracel) gene-modified cellular sheets, also known as pz-cel, as the first and only autologous cell-based gene therapy for the treatment of wounds in adult and pediatric patients with recessive dystrophic epidermolysis bullosa (RDEB). There is no cure for […]
