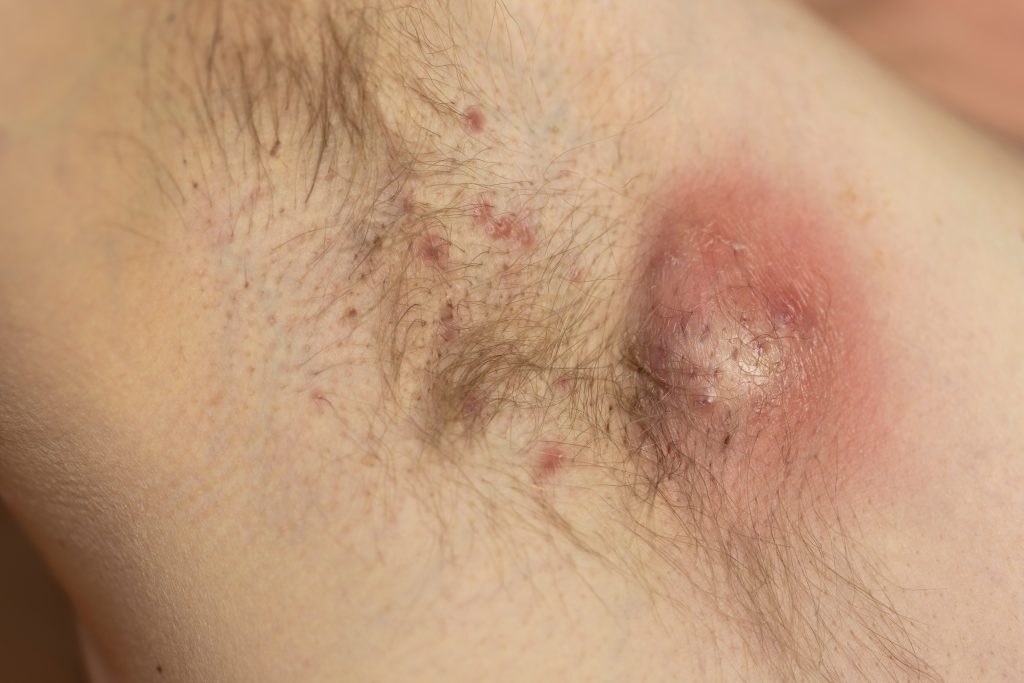
The U.S. Food and Drug Administration (FDA) has accepted the supplemental Biologics License Application (sBLA) for bimekizumab-bkzx (Bimzelx, UCB), an interleukin(IL)-17A and IL-17F inhibitor, for the treatment of adults with moderate-to-severe hidradenitis suppurativa (HS).
In addition, a second sBLA for the Bimzelx 2mL device presentations has also been accepted, according to UCB.
“The most recent sBLA seeks approval for Bimzelx in moderate-to-severe HS, and is aligned to our goal of expanding the reach of Bimzelx to more patients living with IL-17–mediated diseases,” says Emmanuel Caeymaex, Executive Vice President, Immunology Solutions, and Head of U.S. at UCB in a news release. “In addition, the sBLA for the 2mL device presentations aims to offer increased convenience for patients.”
Today, one dose of Bimzelx in moderate-to-severe plaque psoriasis is administered as two 1mL injections. “Approval of the 2mL device presentations would mean that patients would have an alternative one-injection regimen option,” Caeymaex says.
The sBLA in moderate-to-severe HS is supported by data from the Phase 3 BE HEARD I and BE HEARD II studies where Bimzelx demonstrated clinically meaningful improvements in Hidradenitis Suppurativa Clinical Response-50 (HiSCR50) vs. placebo at Week 16, the primary endpoint. A greater proportion of patients treated with Bimzelx vs. placebo also achieved HiSCR75 at Week 16, a key secondary endpoint. In addition, improvements increased for patients in these studies over 48 weeks. The safety profile of Bimzelx was consistent with previous studies with no new safety signals observed.
The two new regulatory milestones represent two of five sBLAs accepted by the FDA for Bimzelx this year, following the previously announced applications in psoriatic arthritis (PsA), non-radiographic axial spondyloarthritis (nr-axSpA) and ankylosing spondylitis (AS).
Bimzelx was approved in the U.S. in October 2023 for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Bimzelx is not approved in the U.S. for the treatment of moderate-to-severe HS, PsA, nr-axSpA, and AS, or for the 2mL device presentation.
In the U.S., the efficacy and safety of Bimzelx for the treatment of moderate-to-severe HS, PsA, nr-axSpA, and AS have not been established and these are investigational indications.