The U.S. Food and Drug Administration (FDA) has given its nod to topical roflumilast foam (Zoryve, Arcutis) for seborrheic dermatitis.
The PDE4 inhibitor is the first topical treatment for seborrheic dermatitis with a new mechanism of action in more than 20 years. Arcutis aims to make the foam widely available via wholesaler and dermatology pharmacy channels by the end of January 2024.
“This is a once-daily non-steroidal treatment that doesn’t have any duration on use,” Patrick Burnett, MD, PhD, chief medical officer at Arcutis, tells TDD.
Topical steroids can only be used in the short term. “This a chronic disease,” he says.
“We also have fantastic itch data that showed a rapid response of itch, the biggest symptom of patients with seborrheic dermatitis.”
Seborrheic dermatitis is not a “by-the-way diagnosis,” says Neal Bhatia, MD, Director of Clinical Dermatology at Therapeutics Clinical Research in San Diego, CA. He took part in the clinical trials that led to the foam’s approval in seborrheic dermatitis.
“Seborrheic dermatitis can be pretty significant for a lot of patients so we really want to get to the heart of treating it safely and thoroughly,” he says in an interview with TDD. “The foam is our new mainstay,”
A Harris Poll conducted on behalf of Arcutis of 300 adults with seborrheic dermatitis and 600-plus health care providers found that individuals with seborrheic dermatitis often experienced a long path to diagnosis, reporting an average of 3.6 years from symptom onset to seeking care, although patients reporting severe disease sought care within 1 year of symptom onset. In contrast, healthcare providers underestimated the time it takes for people with seborrheic dermatitis to receive a diagnosis, reporting an average of just 1.5 years.
These findings suggest that individuals delay seeking care despite experiencing symptoms. In addition, prior to diagnosis, 63% of individuals didn’t think their symptoms were severe enough to warrant medical attention.
The approval is supported by positive results from Arcutis’ Phase 2 and pivotal Phase 3 trials in seborrheic dermatitis. The STudy of Roflumilast foam Applied Topically for the redUction of seborrheic derMatitis (STRATUM) and the Phase 2 (Trial 203) were parallel group, double-blind, vehicle-controlled studies evaluating the safety and efficacy of Zoryve foam 0.3% in seborrheic dermatitis. Together the two studies enrolled 683 adults and adolescents ages 9 years and older.
The STRATUM study met its primary endpoint, with nearly 80% of Zoryve foam-treated individuals reaching the Investigator Global Assessment (IGA) Success rate at Week. In Trial 203, 73% of individuals treated with Zoryve foam achieved IGA Success. IGA Success was defined as an IGA score of “Clear” (0) or “Almost Clear” (1), plus a 2-grade IGA score improvement from baseline at Week 8.
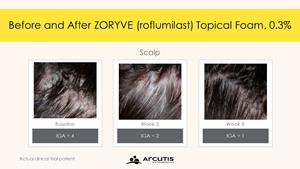

Improvement with Zoryve foam was seen early, with roflumilast demonstrating a statistically significant improvement compared to the vehicle on IGA Success at Week 2, the first time point assessed in STRATUM. In addition, 50.6% of individuals in the Zoryve foam-treated arm reached complete clearance (IGA=0) at Week 8.
The STRATUM study also demonstrated statistically significant improvement over vehicle on all secondary endpoints, including itch, scaling, and erythema. More than 60% of individuals achieved a ≥4-point reduction in itch at Week 8 as measured by Worst Itch-Numerical Rating Score, and significant improvements in itch were also reported at Week 2 and Week 4. Individuals treated with Zoryve foam reported a 28% improvement in itch from baseline in 48 hours.
In addition, more than 50% of individuals treated with Zoryve foam achieved an erythema score of 0, and more than 50% achieved a scaling score of 0, at Week 8. Treatment with Zoryve foam demonstrated a significantly larger improvement in patient-reported outcomes as early as Week 2 as measured through the Dermatology Life Quality Index (DLQI), with improvements maintained through Week 8.
Zoryve foam was well-tolerated with a favorable safety and tolerability profile during up to 52 weeks of treatment. Incidence of Treatment-Emergent Adverse Events (TEAEs) was low and similar between active treatment and vehicle, with most TEAEs assessed as mild to moderate severity. There were no treatment-related Serious Adverse Events (SAEs). Overall, the most common adverse reactions occurring in ≥1% of subjects in the combined Phase 2 and Phase 3 study populations were nasopharyngitis, nausea, and headache.
“In the STRATUM trial, Zoryve foam provided rapid disease clearance as early as Week 2 and significant itch relief in as little as 48 hours. In addition, almost 80% of patients achieved treatment success at Week 8. While multiple factors contribute to seborrheic dermatitis, inflammation, and skin barrier dysfunction play key roles,” says Andrew Blauvelt, MD, MBA, clinical investigator at Oregon Medical Research Center in Lake Oswego, Oregon and investigator on the STRATUM trial, in a news release. “Zoryve has been shown to effectively reduce the signs of inflammation, redness, and scaling in patients with seborrheic dermatitis, and with its unique formulation, Zoryve foam effectively delivers the drug without disrupting the skin barrier and has been shown to be safe and tolerable. Zoryve foam is thus ideally formulated, having the potential to become the new standard of care for seborrheic dermatitis treatment.”


