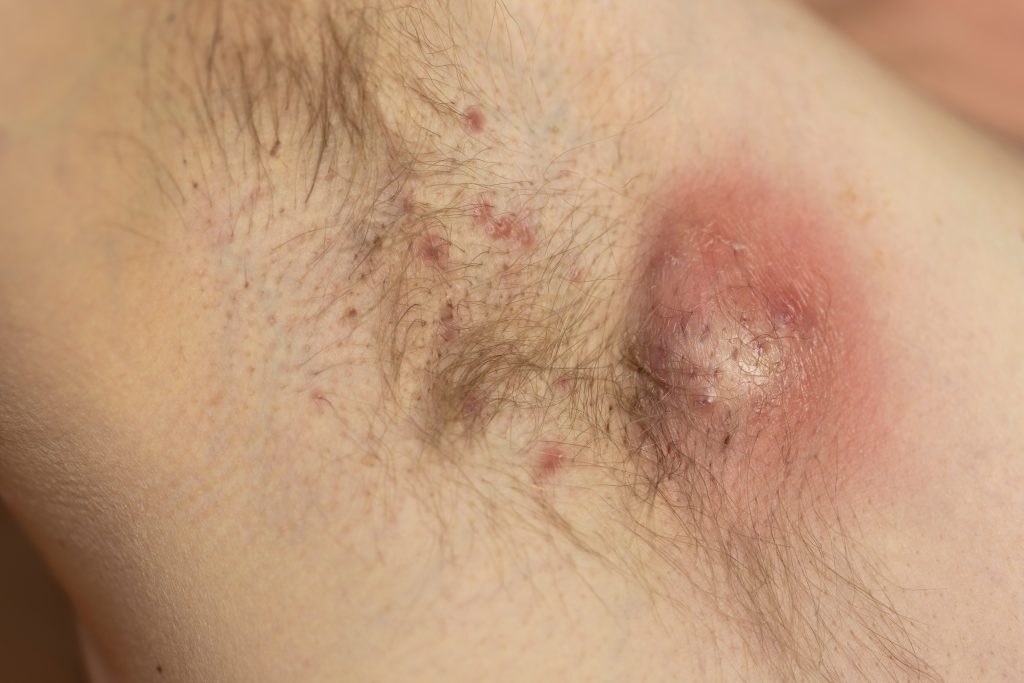
The U.S. Food and Drug Administration (FDA) and E.U. European Medicines Agency (EMA) have greenlighted MoonLake Immunotherapeutics’ Phase 3 Program for the nanobody sonelokimab (SLK) in hidradenitis suppurativa (HS).
Sonelokimab is a novel investigational nanobody for the treatment of inflammatory disease. Sonelokimab inhibits IL-17A and IL-17F by inhibiting the IL-17A/A, IL-17A/F, and IL-17F/F dimers that drive inflammation. Sonelokimab is not yet approved for use in any indication.
The Phase 3 program, named VELA, is expected to enroll 800 patients and in combination with the data from Phase 2 MIRA trial will support both a Biologics License Application (BLA) and E.U. Marketing Authorization Application. The Phase 3 trial design will compare a single SLK dose (120mg) to placebo over a 16-week period, with the placebo group subsequently transitioning to SLK 120mg. The primary endpoint (HiSCR75) and key secondary endpoints read out at week 16. The VELA trial program is designed to run for 52 weeks followed by an open-label extension (OLE). The number of patients, the straightforward trial design, the similarity of protocol to the Phase 2 trial, and the identification of the HS dose, collectively, enhance the clarity of SLK’s clinical development and displays promise for the HS franchise. The first patient expected to be randomized in Q2 2024, and the readout of the primary endpoint is anticipated in mid-2025.
“The favorable response from both the FDA and EMA aligns with the strengths we have consistently highlighted in our trial programs and outlines a clear regulatory path for sonelokimab in hidradenitis suppurativa” says Dr. Jorge Santos da Silva, Chief Executive Officer of MoonLake Immunotherapeutics, in a news release.“ We deeply appreciate the support from both agencies and continue to work swiftly to ramp up the Phase 3 program in HS, named VELA. This represents a crucial step in offering a potential innovative treatment option to patients and their dedicated physicians, addressing a condition that is often under-recognized, under-treated and significantly impacts patients’ lives.”
Additionally, MoonLake plans to have an end-of-Phase 2 meeting with the FDA regarding its psoriatic arthritis (PsA) program in Q2 2024 and begin the Phase 3 program in Q4 2024.
About the MIRA trial
The MIRA trial (M1095-HS-201) is a global, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of the Nanobody sonelokimab, administered subcutaneously, in the treatment of adult patients with active moderate-to-severe hidradenitis suppurativa. The trial recruited 234 patients, with the aim to evaluate two different doses of sonelokimab (120mg and 240mg) with placebo control and adalimumab as an active reference arm. The primary endpoint of the trial is the percentage of participants achieving Hidradenitis Suppurativa Clinical Response 75 (HiSCR75), defined as a ≥75% reduction in total abscess and inflammatory nodule (AN) count with no increase in abscess or draining tunnel count relative to baseline. The trial also evaluated a number of secondary endpoints, including the proportion of patients achieving HiSCR50, the change from baseline in International Hidradenitis Suppurativa Severity Score System (IHS4), the proportion of patients achieving a Dermatology Life Quality Index (DLQI) total score of ≤5, and the proportion of patients achieving at least 30% reduction from baseline in Numerical Rating Scale (NRS30) in the Patient’s Global Assessment of Skin Pain (PGA Skin Pain).