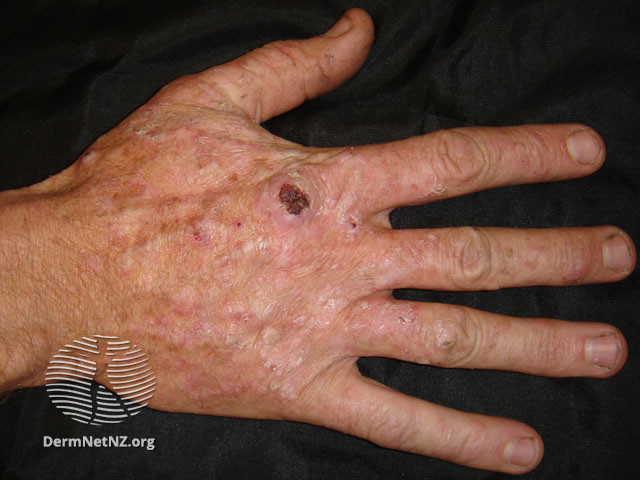
RP1, an investigational oncolytic immunotherapy, may be an effective treatment option for solid organ transplant (SOT) recipients with advanced non- melanoma skin cancer, according to research presented at the American Association for Cancer Research (AACR) Annual Meeting 2024 in San Diego, CA.
Oncolytic immunotherapy uses viruses to target and destroy cancer cells while also stimulating the body’s immune response against the tumor. The viruses are modified so they can selectively infect and replicate within cancer cells, leading to their destruction.
RP1, a modified herpes simplex virus (HSV-1), was well tolerated and achieved an objective response rate of 34.8% and a complete response rate of 21.7% in SOT recipients who had cutaneous squamous cell carcinoma or Merkel cell carcinoma, the Phase Ib/II ARTACUS study showed.
“Organ transplant recipients face an increased risk of non-melanoma skin cancers, and the standard treatment includes immune checkpoint inhibitors. Unfortunately, these therapies can cause rejection of the transplanted organ,” says study author Michael Migden, MD, a professor of dermatology at MD Anderson Cancer Center in Houston. “Data from this study suggest RP1 monotherapy offers a promising possible alternative for this vulnerable patient population.”
The trial followed 27 SOT recipients who had cutaneous squamous cell carcinoma or Merkel cell carcinoma and a median age of 68 years. Tumor biopsies were collected for biomarker analyses and HSV-1 immune status was monitored. After RP1 treatment, participant tumor samples showed an increase in certain immune cells, called CD8+ T cells, entering the tumor, as well as a boost in the production of the immune checkpoint protein PD-L1 within the tumor cells. This suggested increased anti-tumor immune activation.
Adverse effects were minimal, including fatigue, chills and fever, and there was no evidence of transplant rejection.
This study was sponsored by RP1 maker Replimune.