GENERAL DERMATOLOGY
A small (N = 11) retrospective chart review revealed that a majority of patients with SWEET SYNDROME responded to oral dapsone therapy. The median daily dose was 100 mg (range 50 mg to 150 mg). Eight of 9 responders maintained disease control, without flares, for a median time of just under 1 year.
TO READ MORE: Hrin ML, et al. Dapsone as corticosteroid-sparing therapy for Sweet syndrome. J Am Acad Dermatol. 2022;86(3):677-679. doi:10.1016/j.jaad.2021.02.067.
A case of the rare ICHTHYOSIFORM VARIANT OF SARCOIDOSIS was presented. An accompanying literature review disclosed that this form of cutaneous sarcoid is most common in middle-aged Black women and favors the lower and upper extremities. In addition to dermal sarcoidal granulomas, other histological features resemble those found in ichthyosis vulgaris. Of note, over 90% of those with ichthyosiform sarcoid had extra cutaneous organ involvement.
TO READ MORE: Chen HW, et al. Ichthyosiform sarcoidosis: Report of a case and comprehensive review of the literature. Int J Dermatol. 2022;61(4):390-400. doi:10.1111/ijd.15604.

A retrospective case-control study involving 337 VITILIGO patients were given a validated survey to evaluate them for post-traumatic stress. Some 30% of vitiligo patients demonstrated this aberration. Symptoms included: sleep disturbance, intrusive thoughts, situational avoidance, and irritability.
TO READ MORE: Liu JW, et al. Post-Traumatic Stress in Vitiligo Patients: A Neglected but Real-Existing Psychological Impairment. Clin Cosmet Investig Dermatol. 2022;15:373-382. Published 2022 Mar 5. doi:10.2147/CCID.S350000.

According to the authors of this commentary, INPATIENT DERMATOLOGY CONSULTATIONS are not appropriate for patients admitted due to extra-cutaneous disease. Other reasons inpatient dermatology consultations are not appropriate are if a patient is about to be discharged in < 48 hours, for chronic non-life-threatening dermatoses, for hospice care patients, or hemodynamically unstable patients. Examples of inappropriate inpatient consultations include: “rule out skin cancer” or “establish care for psoriasis.”
TO READ MORE: Dobkin H, et al. When are inpatient and emergency dermatologic consultations appropriate? Cutis 2022;109;218-220. doi:10.12788/cutis.0492.
(Editor’s note: Despite this logical algorithm, it is simply easier to obtain dermatological services while the patient is an inpatient (a “captive” audience), rather than rely on the inpatient to follow through after discharge.)
Investigators have perhaps discovered a manner by which to diagnose the severe neurodegenerative disease AMYOTROPHIC LATERAL SCLEROSIS (ALS) at an early stage. Researchers identified TDP-43 protein in the cytoplasm of dermal fibroblasts of individuals with ALS. The same protein was not found in dermal fibroblasts from either healthy controls or from a cohort of patients with Parkinson’s disease and multiple sclerosis.
TO READ MORE: Rubio MA, et al. TDP-43 Cytoplasmic Translocation in the Skin Fibroblasts of ALS Patients. Cells. 2022;11(2):209. Published 2022 Jan 8. doi:10.3390/cells11020209.
On April 19, the U.S. Food and Drug Administration (FDA) issued warning letters to 12 companies selling over-the-counter (OTC) skin lightening products containing HYDROQUINONE as the active ingredient. The FDA does not consider hydroquinone “generally recognized as safe and effective” (abbreviated as GRASE). This stems from regulations included in the CARES Act (Coronavirus Aid, Relief and Economic Security Act), which included not only COVID-19 response efforts, but also updated the method in which certain OTC drugs are regulated.
TO READ MORE: FDA works to protect consumers from potentially harmful OTC skin lightening products. U.S. Food and Drug Administration. April 19, 2022. FDA.gov. Accessed May 17, 2022.
(Editor’s note: OTC hydroquinone has essentially been banned in the United States, and these warning letters indicate that the FDA is taking this seriously.)
ACNE

A group in India pointed out that MASK-RELATED ACNE is more common and more severe in skin of color. This relates to mask-induced increased skin temperature and humidity. The latter two factors lead to excessive sebum production (especially squalene) and swelling of keratinocytes with obstruction of pilosebaceous ducts.
TO READ MORE: Raju SP, et al. Mask Acne in Skin of Color: A Significant Dermatological Condition Amidst the COVID-19 Pandemic. J Clin Aesthet Dermatol. 2022;15(4):44-48.
ROSACEA
Rosacea patients who responded to subantimicrobial dose DOXYCYCLINE plus topical metronidazole 1% gel were randomly assigned to either receive placebo or continue on subantimicrobial dose doxycycline. After 40 weeks, those who remained on the modified-release doxycycline fared better, as their relapse rate was only 13.8% compared to 27.7% in the control group. Subantibiotic dose doxycycline seems to be a viable long-term management strategy for rosacea.
TO READ MORE: Del Rosso JQ, et al. Long-term inflammatory rosacea management with subantibiotic dose oral doxycycline 40 mg modified-release capsules once daily. Dermatol Ther. 2022;35(1):e15180. doi:10.1111/dth.15180.
PEDIATRIC DERMATOLOGY
In the pediatric population, LICHEN PLANUS (LP) can present in a wide variety of ways. Notably, linear LP is more common and oral lesions less common, when compared to adults. Topical and oral corticosteroids are the most effective therapeutic interventions.
TO READ MORE: Merhy R, et al. Pediatric lichen planus: a systematic review of 985 published cases. Int J Dermatol. 2022;61(4):416-421. doi:10.1111/ijd.15678.

TMB-001, topical formulation of isotretinoin in a specialized delivery system (Timber Pharmaceuticals), has been granted fast track designation by the FDA for the treatment of congenital x-linked recessive ichthyosis and autosomal recessive lamellar ichthyosis. Earlier studies involving this agent were positive.
TO READ MORE: Timber Pharmaceuticals Announces Fast Track Designation Granted by FDA for TMB-001 in SEVERE SUBTYPES OF CONGENITAL ICHTHYOSIS. Timber Pharmaceuticals press release. April 28, 2022. Accessed May 17, 2022.
PSORIASIS
Imsidolimab, a humanized anti IL-36 receptor monoclonal antibody, is entering phase 3 studies for the acute and long-term management of generalized PUSTULAR PSORIASIS. Both efficacy and safety will be evaluated.
SOURCE: Gudjonsson JE, et al. Imsidolimab in the treatment of adult subjects with generalized pustular psoriasis: Design of a pivotal Phase 3 clinical trial and a long-term extension study. Presented at the American Academy of Dermatology Annual Meeting. Boston, Massachusetts. March 25-29, 2022. Poster 34617
A retrospective review of 16 melanoma patients (10 invasive and 6 in-situ) treated for psoriasis with APREMILAST for 36 months did not demonstrate any melanoma recurrence. Literature review similarly verifies safety of apremilast therapy in melanoma patients.
TO READ MORE: Gambardella A, et al. Is Apremilast a Safe Option in Patients with History of Melanoma? A Case Series and a Review of the Literature. J Clin Aesthet Dermatol. 2022;15(2):23-25.
The presence of circulating cardiac high-sensitivity troponin I (cTnI) and N-terminal pro-brain-type natriuretic peptide (NT-proBNP) in patients with psoriasis and psoriatic arthritis correlated with carotid artery plaque formation and incident cardiovascular events (MI, stroke). These CARDIAC BIOMARKERS proved as good as, but not superior to, the Framingham Risk Score, which takes into account age, sex, smoking habits, systolic blood pressure, hypertension treatment, diabetes, total cholesterol and high-density lipoprotein cholesterol levels. The prognostic utility of this information remains to be determined.
TO READ MORE: Colaco K, et al. Association of Cardiac Biomarkers With Cardiovascular Outcomes in Patients With Psoriatic Arthritis and Psoriasis: A Longitudinal Cohort Study [published online ahead of print, 2022 Mar 8]. Arthritis Rheumatol. 2022;10.1002/art.42079. doi:10.1002/art.42079.
A Korean cross-sectional study involved 37,999 patients with PALMOPLANTAR PUSTULOSIS (PPP), 332,279 psoriatics patients and 365,415 dyshidrosis patients. Compared to dyshidrosis, PPP had a stronger association with cardiometabolic disease, inflammatory arthritis, and autoimmune diseases (including vitiligo). When PPP was compared to psoriasis, the risks of ankylosing spondylitis and Graves disease were higher.
TO READ MORE: Kim DH, et al. Risks of Comorbidities in Patients With Palmoplantar Pustulosis vs Patients With Psoriasis Vulgaris or Pompholyx in Korea [published online ahead of print, 2022 Apr 27]. JAMA Dermatol. 2022;e221081. doi:10.1001/jamadermatol.2022.1081.
ATOPIC DERMATITIS
Among prior DUPILUMAB NONRESPONDERS, EASI-75 was obtained in 80.0% and 67.7% in those who received abrocitinib 200 mg and 100 mg daily, respectively. The higher daily abrocitinib dose also resulted in 77.3% of recipients achieving a > 4-point improvement in Peak Pruritus Numerical Rating Scale, while this measure of itch improved in 37.8% of those who received the lower abrocitinib dose.
TO READ MORE: Shi VY, et al. Phase 3 Efficacy and Safety of Abrocitinib in Adults with Moderate-to-Severe Atopic Dermatitis After Switching from Dupilumab (JADE EXTEND) [published online ahead of print, 2022 Apr 16]. J Am Acad Dermatol. 2022;S0190-9622(22)00608-9. doi:10.1016/j.jaad.2022.04.009.
A retrospective study of a large electronic record database, including only patients 60 to 99 years old, disclosed a 27% increased RISK OF DEMENTIA among those with atopic dermatitis. Among those with atopic eczema, the incidence of dementia was 57/10,000 person-years (95% CI, 56-59), compared with 44/10,000 person-years (95% CI, 44-45) in those without eczema. Perhaps the chronic inflammation associated with atopic dermatitis predisposes to neurodegenerative disease.
TO READ MORE: Magyari A, et al. Adult atopic eczema and the risk of dementia: A population-based cohort study [published online ahead of print, 2022 Mar 30]. J Am Acad Dermatol. 2022;S0190-9622(22)00541-2. doi:10.1016/j.jaad.2022.03.049.

COSMETIC DERMATOLOGY
A known side effect of botulinum toxin injections in/near the glabella is EYELID PTOSIS. While this can be reversed by intraocular drops, this case report demonstrated the same effect using brimonidine 0.33% gel (Mirvaso). There is less risk of systemic absorption utilizing the topical gel to correct this transient cosmetic adverse event.
TO READ MORE: Alotaibi GF, et al. Eyelid ptosis following botulinum toxin injection treated with brimonidine 0.33% topical gel. JAAD Case Rep. 2022;22:96-98. Published 2022 Jan 31. doi:10.1016/j.jdcr.2022.01.019.
Beware! Q-switched 1064nm LASER HAIR REMOVAL on the upper or lower lip can cause paradoxical and unwanted darkening of previously placed lip and lip border tattoos.
TO READ MORE: Al-Jasser MI, et al. Lip Tattoo Darkening After Q-Switched Laser Hair Removal Followed by Significant Spontaneous Improvement. J Clin Aesthet Dermatol. 2022;15(4):10.
HAIR AND NAILS
CHEMOTHERAPY-INDUCED PARONYCHIA is most commonly associated with administration of both classes of epidermal growth factor receptor (EGFR) inhibitors (examples: cetuximab, panitumumab, eroltinib, gefitinib, afatinib, and osimertinib) and taxane agents (examples: paclitaxel, docetaxel). The great toenail is most commonly affected, and the disease process typically starts 1 to 2 months after initiation of chemotherapy. Topical therapies include dilute bleach soaks, corticosteroids, and calcineurin inhibitors. These may be combined with oral tetracycline derivatives for severe cases.
TO READ MORE: Gupta MK, et al. Review of chemotherapy-associated paronychia. Int J Dermatol. 2022;61(4):410-415. doi:10.1111/ijd.15740.
SCALP DYSESTHESIA (pain, burning, stinging without observable abnormality) may be due to stroke, brain tumor, vascular malformations, demyelinating disease, radicular compression, and underlying meningioma.
TO READ MORE: McConnell RD, et al. Scalp dysesthesia, more than skin deep. JAAD Case Rep. 2022;22:82-84. Published 2022 Jan 6. doi:10.1016/j.jdcr.2021.12.001
HIDRADENITIS SUPPURATIVA
A retrospective cohort study sourced from a large health record database compared 37,702 hidradenitis suppurativa patients to a similar number of matched controls. Compared to the control group, HS patients were at increased risk for rheumatoid arthritis, inflammatory bowel disease, myocardial and cerebral infarction, malignancy, and thromboembolic events. Notably, HS patients had increased C-reactive protein levels and higher erythrocyte sedimentation rates. CRP levels >10mg/L were predictive of a higher mortality rate.
SOURCE: Parthasarathy V, et al. C-reactive protein levels and circulating blood inflammatory markers are associated with poor outcomes in hidradenitis suppurativa patients: A multi-center cohort study. Presented at the American Academy of Dermatology Annual Meeting. Boston, Massachusetts. March 25-29, 2022.
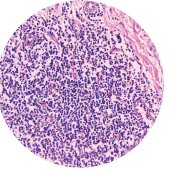
A single-arm, prospective study, including 43 patients with Hurley stage I to III hidradenitis, evaluated success of DEROOFING as a means of disease management. Of the 123 lesions which were deroofed, only 7% had any recurrence 3 months post-operatively, and 93% reported no drainage at the surgical site.
SOURCE: Vu TT, et al. Prospective study of surgical deroofing in the management of hidradenitis suppurativa. Poster. Presented at the American Academy of Dermatology Annual Meeting. March 25-29, 2022. Boston.
(Editor’s note: Tracing out HS tracks with a probe, deroofing them, and then allowing healing by secondary intent is NOT a new concept. I did this when I was a resident in the 1970s!)
CUTANEOUS ONCOLOGY, SURGERY AND LASERS
Nearly 444,500 solid organ transplant recipients identified from the Scientific Registry of Transplant Recipients were analyzed for incident NONKERATINOCYTE SKIN CANCER. From this cohort, 2380 transplant recipients were diagnosed with nonkeratinocyte skin cancer. Malignant melanoma constituted 61.8% of these lesions. Compared to the general population, the transplant recipients also had an increased risk of developing Kaposi sarcoma, Merkel cell carcinoma, and sebaceous carcinoma. Risk factors associated with nonkeratinocyte skin cancers in solid organ transplant patients included: older age at the time of transplant, prolonged time since initial transplant, male gender, and excessive ultraviolet light exposure.
TO READ MORE: Sargen MR, et al. Spectrum of Nonkeratinocyte Skin Cancer Risk Among Solid Organ Transplant Recipients in the US. JAMA Dermatol. 2022;158(4):414-425. doi:10.1001/jamadermatol.2022.0036.
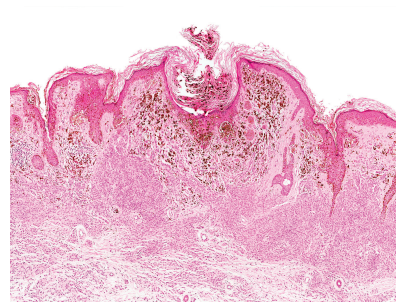
Based on the results of a large-scale observational study, investigators concluded that SCREENING OLDER PATIENTS FOR MELANOMA, especially men, may be cost-effective. Moreover, in this primary care based study, melanomas detected during specific screening sessions tended to be in situ or thin invasive (1 mm or less in thickness) compared to those detected in patients who had not attended one or more screening sessions.
TO READ MORE: Matsumoto M, et al. Five-Year Outcomes of a Melanoma Screening Initiative in a Large Health Care System [published online ahead of print, 2022 Apr 6]. JAMA Dermatol. 2022;e220253. doi:10.1001/jamadermatol.2022.0253.
INFECTIOUS DISEASES
VACCINES TO PREVENT HPV-RELATED DISEASE (ano-genital tract squamous cell carcinoma and external genital warts) have been available since 2006. A comprehensive literature review disclosed that, among patients aged 9 to 26, those who received motivational or reminder text messages had higher initial vaccination rates and more timely subsequent vaccinations than comparable individuals who received no message.
TO READ MORE: Khuwaja SS, et al. Increasing HPV Vaccination Rates Using Text Reminders: An Integrative Review of the Literature [published online ahead of print, 2022 Mar 11]. J Pediatr Health Care. 2022;S0891-5245(22)00027-X. doi:10.1016/j.pedhc.2022.02.001
COVID-19
One hundred forty-eight COVID patients without a PRESSURE ULCER who were admitted to the ICU for >24 hours were followed. Thirty-seven developed a pressure ulcer. Risk factors included higher body mass index (BMI), longer time spent in ICU, and evidence of consumptive coagulopathy. Notably, pressure ulcers were about 12 times more likely to develop in patients with a D-dimer level of >0.5ug/mL and a fibrinogen level of <2.0mg/mL on admission.
TO READ MORE: McLarney BD, et al. Predictors of COVID-19 disease severity augment the Braden scale in the prediction of pressure ulcer development among COVID-19-positive intensive care unit patients: A case-control study [published online ahead of print, 2022 Feb 25]. J Am Acad Dermatol. 2022;S0190-9622(22)00099-8. doi:10.1016/j.jaad.2022.01.021.
In a systematic literature review, investigators found that long COVID may be accompanied by cutaneous manifestations. Not surprisingly, the most common was alopecia (probably telogen effluvium). However, of the 236 patients covered in this review, pernio-like lesions were seen in 4.2%, a maculo-papular eruption in 1.7%, persistent urticaria in 0.8%, and vesicular, papulosquamous, and purpuric lesions in 0.4% each. Lesions could arise as late as 6 months after initial COVID diagnosis and could last from as little as a week to as long as 240 days following COVID symptom resolution. Neither the mechanism nor the prognostic significance of such skin lesions is known.
SOURCE: Grover A, et al. Long-term cutaneous manifestations in COVID-19 patients: A systematic review. Presented at the American Academy of Dermatology Annual Meeting. Boston, Massachusetts. March 25-29, 2022.
The U.S. Food and Drug Administration issued an emergency use authorization (EUA) for a COVID-19 diagnostic test that detects chemical compounds in breath samples associated with SARS-CoV-2 infection. The test, INSPECTIR BREATHALYZER, can be performed medical offices, hospitals, and mobile testing sites using an instrument about the size of a piece of carry-on luggage. In a large study of 2,409 individuals, the test was shown to have 91.2% sensitivity and 99.3% specificity.
TO READ MORE: Coronavirus (COVID-19) Update: FDA Authorizes First COVID-19 Diagnostic Test Using Breath Samples. FDA Press Release. April 14, 2022. Accessed May 17, 2022.


