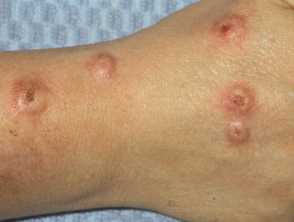
More than 80% of nemolizumab-treated patients with prurigo nodularis (PN) experienced improvement in itch, and more than 60% achieved clear or almost-clear skin, according to one-year data from an interim analysis of the OLYMPIA long-term extension (LTE) study presented at the 2024 American Academy of Dermatology Association (AAD) Annual meeting in San Diego.
In addition, results from the phase III ARCADIA clinical trials in atopic dermatitis (AD) found that nemolizumab-treated patients who responded at 16 weeks maintained skin and itch responses through to week 48, even when dosing was reduced from every four to every eight weeks.
“These data further confirm the efficacy and safety of nemolizumab in both AD and PN,” says TDD Editorial Advisory Board Member Jonathan Silverberg, MD, PhD, MPH, a Professor of Dermatology at The George Washington University School of Medicine and Health Sciences in Washington, DC, in an interview. “In particular, they provide important data to support the long-term use of nemolizumab for management of chronic AD and PN.”
Nemolizumab is a first-in-class investigational monoclonal antibody specifically designed to inhibit IL-31 signaling to provide safe and rapid relief from the most burdensome symptom of both skin conditions: itch.
The U.S. Food and Drug Administration (FDA) recently accepted Galderma’s Biologics License Applications for nemolizumab for the treatment of patients with prurigo nodularis (PN) and for adolescents and adults with moderate to severe atopic dermatitis (AD). In addition, the European Medicines Agency has also accepted the Marketing Authorization Applications for nemolizumab in prurigo nodularis and atopic dermatitis.
The FDA has granted nemolizumab Priority Review for prurigo nodularis. This follows its designation as a Breakthrough Therapy for the treatment of pruritus associated with prurigo nodularis, originally granted in December 2019 and reconfirmed in March 2023.
OLYMPIA LTE Update
Building on the results of OLYMPIA 1 and OLYMPIA 2, the OLYMPIA open-label LTE study is an ongoing 184-week trial to evaluate the long-term efficacy and safety of nemolizumab monotherapy (without topical corticosteroids) in patients with moderate to severe prurigo nodularis who had received nemolizumab in phase II and III lead-in trials as well as those who had previously received placebo (nemolizumab-naïve).
Results from an interim analysis of the OLYMPIA LTE study demonstrated that nemolizumab treatment provided continuous improvement in skin lesions and itch up to week 52. Specifically, 69% of those who had received continuous nemolizumab treatment and 65% of nemolizumab-naïve patients had reached clearance or almost-clearance of skin lesions, as measured on the Investigator’s Global Assessment (IGA) score. Fully 89% of those who had received continuous nemolizumab treatment and 83% of nemolizumab-naïve patients achieved a significant response on itch intensity as measured by an at least four-point improvement on the peak-pruritus numerical rating scale (PP-NRS), Sleep disturbance, as measured by the sleep disturbance numerical rating scale (SD-NRS), also continued to improve, as well as quality of life, as measured by the Dermatology Life Quality Index (DLQI), the study showed.
Nemolizumab-naïve patients rapidly achieved an at least four-point improvement in itch intensity, as measured by the PP-NRS, as early as week four, consistent with the continuous-nemolizumab cohort. No new safety signals were observed in this trial.
ARCADIA 1 and ARCADIA 2 Update
New results from ARCADIA 1 and ARCADIA 2 in AD showed that the majority of patients who had clinical responses to skin lesions* at week 16 in ARCADIA 1 maintained skin and itch responses at week 48 when receiving nemolizumab every four weeks (Q4W) or every eight weeks (Q8W) in the maintenance phase. Fully 62% of those receiving nemolizumab Q4W and 60% of those receiving nemolizumab Q8W maintained clearance or almost-clearance of skin lesions, when assessed using the IGA score, compared to 50% receiving placebo, the study showed.
The Eczema Area and Severity Index (EASI)-75 score was also maintained in 76% of those receiving nemolizumab—both Q4W and Q8W—compared to 64% receiving a placebo. Itch response, as measured by an at least four-point improvement in weekly average PP-NRS score, was similarly maintained in the majority of patients receiving nemolizumab—both Q4W and Q8W—compared to placebo
Response in sleep and quality of life, as measured by the SD-NRS and DLQI scales, respectively, were also well maintained in the study. The safety profile was consistent across treatment arms and most treatment-related adverse events were non-serious and mild/moderate in intensity.
PHOTO COURTESY OF DermNet NZ