A Pipeline Treatment for Molluscum Contagiosum
With John C. Browning, MD
Dr. John C. Browning discusses phase 3 data and possible uses in practice for investigational Berdazimer 10.3% gel in the treatment of molluscum contagiosum.

“The FDA has not approved anything to treat molluscum contagiosum, so there is not a recognized treatment or standard of care for this disorder,” said John C. Browning, MD, of Texas Dermatology and Laser Specialists in San Antonio, Texas.
“You will hear some dermatologists say ‘don’t treat’ because molluscum contagiosum is self-limited, but this advice is frustrating to parents as molluscum can lead to scarring. Others will treat with cryotherapy, laser therapy, or cantharidin. But these treatment modalities are not consistent, and they don’t always work.”
In the Pipeline
Novan announced that preliminary phase 3 data was presented at the 2022 Winter Clinical Dermatology Conference on Berdazimer 10.3% gel, which could be a first-in-class topical controlled-nitric oxide (NO)-release medication approved to treat molluscum contagiosum.
Dr. Browning, an investigator on the drug’s phase 2 and 3 studies, said Berdazimer 10.3% is novel for reasons other than potentially being the first FDA approved medication for molluscum contagiosum.
“[Berdazimer 10.3%] has a novel mechanism of action because it harnesses the antiviral effects of nitric oxide by combining nitric oxide in the Berdazimer molecule with a hydrogel. This allows release of the nitric oxide with antiviral properties on the molluscum,” he said.
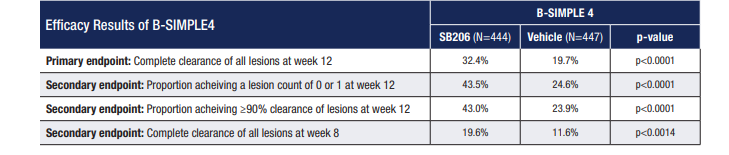
The phase 3 B-SIMPLE4 trial was a multicenter, double-blind, vehicle-controlled study of 891 patients randomized to receive Berdazimer 10.3% or vehicle only. Patients were treated for up to 12 weeks, with the primary endpoint being the proportion of patients with complete clearance of all treatable molluscum lesions at week 12.
“In the phase 3 trial, 32.4% in the Berdazimer group showed clearance by week 12, whereas only 19.7% in the vehicle alone group showed clearance by week 12. Even though 32.4% versus 19.7% doesn’t seem like a huge difference, it is statistically significant,” Dr. Browning said. (See Table: B-SIMPLE4 Efficacy Results)
“If you look at acne or atopic dermatitis trials for topical medications, you’ll see these same types of numbers where there is improvement in the vehicle group as well as the treatment group. That’s because some people will get better, no matter what, in the vehicle group, especially in self-limited conditions or disorders with waxing and waning….”
To have almost a third of patients clear with treatment is an encouraging finding, according to Dr. Browning, especially given that this number does not include patients in the treatment group who may have seen a reduction in molluscum lesions overall or clearance of troublesome areas but didn’t meet the study endpoint because they were not clear by week 12.
“I think there will be excitement among dermatologists to have something topically that they can prescribe to treat molluscum,” Dr. Browning said.
“Dermatologists may still use the modalities that they’re comfortable with. They may still want to use cantharidin or cryotherapy to treat molluscum. But they might also have a topical that they can prescribe to patients, so they’re not necessarily having to bring people back in a month or six weeks for another treatment.”
Patient Education
“The most common adverse events [in this study] was application site pain and erythema. Most cases were mild, but it’s something that requires education because if you are not expecting to see the molluscum get red or perhaps even painful, that could derail patients from using it properly,” Dr. Browning said.
“This same type of education is needed when prescribing topical retinoids to treat acne. If we don’t adequately counsel our patients or the families about the expected irritation from topical retinoids, then they may discontinue therapy. I just think proper education will be very helpful so that people understand that there will be some redness and irritation, which is expected.”
Real World Potential
If approved, Dr. Browning said he will likely prescribe the topical to treat molluscum on the neck and face, especially in areas where he would not want to use cryotherapy or cantharidin.
“I could see using Berdazimer on more sensitive areas, like under the arm or sensitive areas. I can also see it being used as adjunctive therapy to destructive therapy. I use a lot of cantharidin in my office and can imagine that I would still use cantharidin, but I might also prescribe Berdazimer to be used at home for any of the molluscum bumps that don’t resolve with cantharidin or any new ones that form,” he said.
“One other thing to consider is that there are patients who either don’t want to undergo a procedure because they don’t want any kind of blistering or side effects from the procedure, or they don’t want to pay an out-of-pocket deductible required by their insurance, so they may be more interested in a topical treatment.”
By Lisette Hilton
Disclosure: Dr. Browning is a researcher for the company but has no other ties.