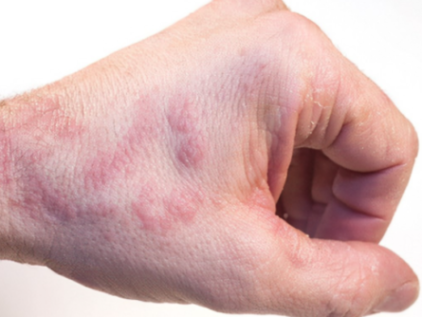
Expanding the AD Treatment Armamentarium
With Lawrence F. Eichenfield, MD

New topical nonsteroidal improves signs and symptoms of AD
The FDA approval of ruxolitinib 1.5% cream (Opzelura, Incyte) for the treatment of atopic dermatitis (AD) provides dermatologists with a new nonsteroidal option offering both anti-inflammatory and anti-pruritic activity.
“Ruxolitinib is the first janus kinase (JAK) inhibitor approved for treating AD and the first approved topical JAK inhibitor. Ruxolitinib blocks JAK1 and JAK2 and provides dual benefits for treating AD because the JAK-Stat pathway mediates inflammation and may also mediate itch,” said Lawrence F. Eichenfield, MD, Professor of Dermatology and Pediatrics, University of California, San Diego.
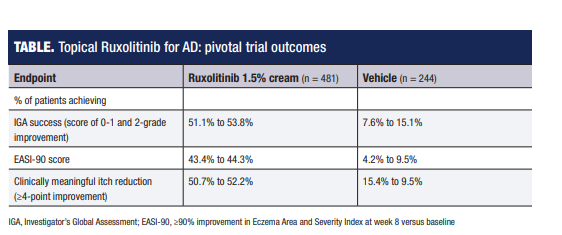
“In two 8-week phase 3 studies, over 50% of patients using ruxolitinib 1.5% cream twice daily achieved treatment success defined as an Investigator’s Global Assessment rating of ‘clear or almost clear’ with at least a 2-step improvement from baseline. Mean itch score for the entire cohort averaged around 5 at baseline on a scale of 0 to 10, and among patients with an itch score of at least 4 at baseline, more than 50% had at least a 4-point decrease at week 8. Significant itch reduction versus vehicle control was even seen within 12 hours after the first use.”
Treatment with topical ruxolitinib was also safe and well-tolerated in the pivotal trials.
“Rates of application site burning and pruritus, which were the most common treatment-related adverse events, were very low (<1%) in the ruxolitinib 1.5% cream group and lower than in the control group,” Dr. Eichenfield said.
“The prescribing information contains Black Box warnings about adverse events, but they mostly reflect experience with oral JAK inhibitors. Pharmacokinetics data show no evidence of systemic accumulation with topical ruxolitinib application over time.”
Dr. Eichenfield added that ruxolitinib cream is indicated for short-term and non-continuous chronic treatment for patients whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable, and it is recommended to be applied to no more than 20% of the body surface area.

“The average body surface area of involvement for patients in the phase 3 pivotal trials was just under 10%, and the treatment was used for 8 weeks,” he said.
“Data from patients participating in an extension of the phase 3 trials offer evidence that could support the safety and efficacy of longer-term use. In the 1-year extension study, patients used ruxolitinib when their AD was not clear or almost clear, and stopped it when it was. The extent of treatment in this ‘real-life protocol’ was variable over the year, but no new safety signals emerged.”
Role in the AD treatment algorithm
Although topical ruxolitinib was studied as monotherapy in the phase 3 trial, Dr. Eichenfield expects that dermatologists will likely also be using it in combination with other topical agents or even systemic treatments for AD. He suggested it could be considered as the initial topical treatment, but expects it is unlikely that dermatologists will routinely choose topical ruxolitinib instead of a topical corticosteroid.
“It is not very practical to abandon traditional therapeutic approaches, and topical triamcinolone and other steroids are used widely and successfully in regimens of AD patient care. Of course, topical steroids must be used properly and have limitations,” Dr. Eichenfield said.
“As a topical nonsteroidal medication, however, ruxolitinib should prove very helpful when used early in the AD treatment regimen for ‘delicate areas’ that are at higher risk of steroid atrophy or to complement or replace other topical treatments.”
Topical ruxolitinib has not been directly compared against other nonsteroidal treatments for AD. In a phase 2 randomized trial, outcomes were a little better among patients treated with ruxolitinib cream than controls using 0.1% triamcinolone cream.
“Triamcinolone, a mid-strength topical steroid, is a workhorse for treating AD. The results of the phase 2 trial suggest that ruxolitinib may be more efficacious than topical calcineurin inhibitors or crisaborole (Eucrisa, Pfizer),” Dr. Eichenfield said.
Results from the phase 3 AD studies are published.1
By Cheryl Guttman Krader
_____________________________________________________________________________________________________________________________________________________
Reference:
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85(4):863-872. doi:10.1016/j.jaad.2021.04.085.
_____________________________________________________________________________________________________________________________________________________________________________________
Disclosure
Dr. Eichenfield was an investigator in the ruxolitinib cream clinical trials and has served as a consultant or investigator for Abbvie, Almirall, Aslan, Dermavant, Lilly, Forte, Galderma, Incyte, Leo, Novartis, Ortho Dermatologics, Otsuka, Pfizer and Sanofi-Regeneron.
IGA, Investigator’s Global Assessment; EASI-90, ≥90% improvement in Eczema Area and Severity Index at week 8 versus baseline