By Lisette Hilton | Reviewed by Wm. Philip Werschler, MD, FAAD, FAACS

There is plenty of chatter in aesthetic medicine about
the potential role regenerative therapies will play.
Wm. Philip Werschler, MD, FAAD, FAACS, revealed at Maui Derm 2021 that regenerative medicine already has taken hold in some aesthetic practices, and with time it will significantly impact what aesthetic providers do and how they do it. “I think there is a lot of interest, but people don’t really know much. Dermatologists have not been drivers for the most part in regenerative aesthetics, yet we are the largest potential market,” Dr. Werschler said.
Definitions
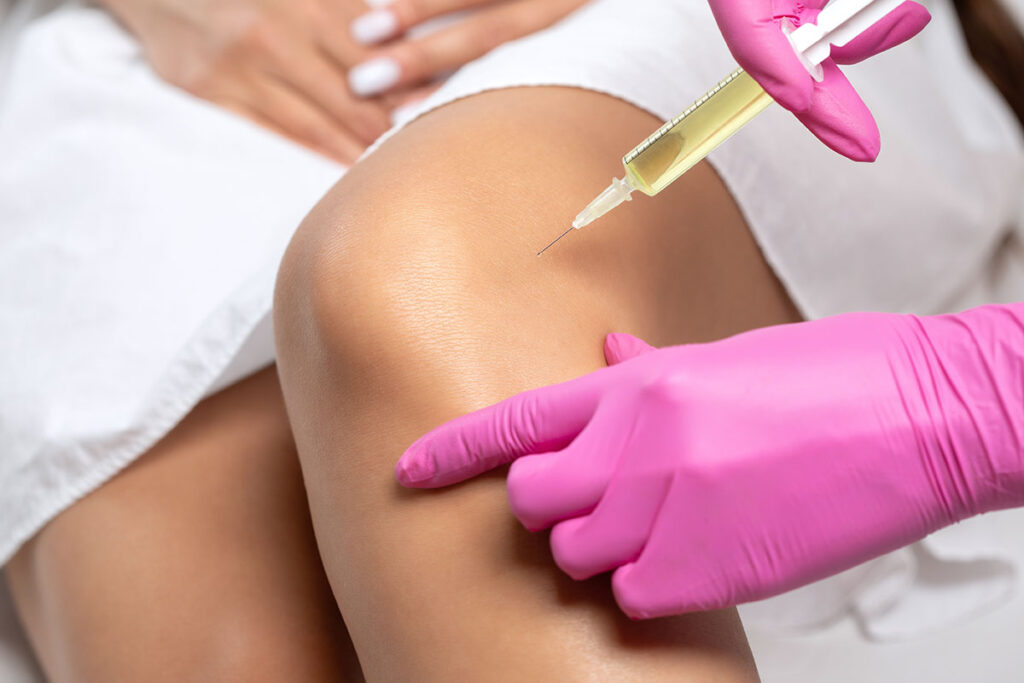
Regenerative aesthetic medicine (RAM) is a branch of regenerative medicine, which is widely practiced in orthopedics, neurology, and even plastic surgery.
“It has been very slow, next to impossible, to get any kind of reimbursement for regenerative procedures. Insurance does not pay for them. All these stem-cell injections into arthritic knees and platelet-rich plasma (PRP) treatments for bad backs—insurance doesn’t pay for those,” Dr. Werschler said.
“The aesthetic marketplace is the most fertile marketplace to monetize regenerative medicine and hence the term ‘regenerative aesthetic medicine.’”
Moving past the word salad to get to the meat
One of the problems is consistency. This area of medicine and aesthetics can best be described as a “word salad,” according to Dr. Werschler, of terms and (worse) acronyms, that mean different things to different people.
Dr. Werschler starts with 2 generally recognized and widely used regenerative aesthetic medicine therapies: PRP and stem cells.
“But what does “stem cell” mean? Most people think that PRP has stem cells. There are no stem cells in PRP. PRP has platelets,” Dr. Werschler said. “Even within PRP, you have platelet-rich plasma, platelet-poor plasma, platelet-rich plasma derivatives, platelet-rich fibrin, platelet-rich fibrin matrix. You take all of those and that is just one technique.”
Using the term stem cells is as broad as saying something is a “car.” There are different kinds of stem cells, including pluripotent, mesenchymal, induced, bone marrow, and adipose-derived. Dermatologists are most likely to use adipose-derived stem cells, according to Dr. Werschler.
Market size, predictions
Lacking concrete numbers defining the regenerative medicine market’s size, Dr. Werschler researched and presented global aesthetic market breakdown information from various sources.
“Market reports indicate in pie charts that a big chunk is energy devices, and a big chunk is neurotoxins and fillers, both of which probably make up 40% of the market. Just about the thinnest slice is emerging therapies, stem cells, PRP and they lumped threads in, but the growth rate is 23%, which is the corporate average growth rate, or CAGR. Research reports show massive growth rates for regenerative medicine from 2018 to 2026 and from 2018 to 2024,” he said. “The relative growth of these procedures, no matter who you talk to or what dataset you look at, is faster relative growth than for established energy-based devices and injectable categories.”

The market growth might not be so evident to dermatologists because companies driving the regeneration market tend not to be traditional dermatology pharmaceutical brands, although Allergan is listed as 1 of 5 global companies involved in consolidation of the market. Others are in the orthopedic and in areas focused on growing body parts and more, according to Dr. Werschler.
There also are smaller companies looking to get their share of the growing market and maybe the opportunity to sell to bigger players. One that many dermatologists know, Suneva, maker of Bellafill, is marketing itself as “shaping the future of regenerative medicine.”
FDA’s position: Watch your step
The FDA appears to be learning how to navigate its oversight of regenerative medicine. The FDA does not really say what providers can and cannot do until after the fact, according to Dr. Werschler. “What the FDA will do is give guidance,” he said.
Regenerative medicine falls under the 21st Century Cures Act, signed into law December 13, 2016. The law is designed to accelerate medical product development and innovation. The FDA considers adipose tissue to be a structural tissue and states that minimal manipulation can be used in patients without FDA premarket approval, but more than minimal manipulation requires an approved investigational New Drug Application.
The FDA does not define exactly what minimal manipulation is, according to Dr. Werschler. For example, a provider who takes blood and spins it down to concentrate platelets for PRP ends up with 4 or 5 times the normal physiologic concentration. Then, the provider injects platelets into tissue. Even though that provider is altering the structure and function of the platelets, the FDA considers that to be minimal manipulation. Doing more than that is where the regulatory trouble starts. Stromal vascular fraction, for example, is a concentrated source of adipose-derived stem cells and is regulated by the FDA as a drug/biological product. It is a false premise to assert that a product derived from a person’s own body and then manipulated and reinserted for a use other than what it did in its original location is not subject to FDA regulation, according to Dr. Werschler.
Procedures that fall under the FDA’s minimal manipulation guidelines need to be registered with the agency, not approved.
“FDA regulates it from an infectious disease standpoint. In other words, they oversee it to make sure there is no transmission in the handling, in the storage, in the reinjection, or the use that puts the patient at risk for transmission of an infectious disease,” Dr. Werschler said. “If you manipulate it a little bit more than that, you have to apply for a biologics license….”
Dermatologists need to be aware of the FDA’s guidance and realize that when industry representatives tell them that regenerative products and techniques are FDA-approved, they are not, according to Dr. Werschler.
“The problem is that you have decades and decades of precedence when you look at regular drugs, regular biologics, and devices. But this regenerative stuff is all new, and the FDA does not know what to do with it,” Dr. Werschler said. So, for now the FDA focuses on safety. “We decide where the road ends and where the grass starts,” Dr. Werschler said. “That’s the regulatory side of things. I would say it is somewhere between very muddy and murky water. It is not clear. If I take an antibiotic that is not approved and give it to patients to treat infections, that is black and white. I could get fined, lose my license, go to jail. There is very little black and white in the world of regenerative medicine.”