Contrary to concerns regarding malignancy, venous thromboembolism, and major adverse cardiac events, the use of Janus kinase (JAK) inhibitors is not associated with a significant increase in the overall risk for these conditions in patients with atopic dermatitis (AD), according to a systematic review and meta-analysis, published in the Journal of the European Academy of Dermatology & Venereology.
Recently, 10 JAK inhibitors have been approved by the US Food and Drug Administration (FDA) to treat diseases like rheumatoid arthritis, ankylosing spondylarthritis, COVID-19, and AD, among others. However, the FDA has also issued a boxed warning for JAK inhibitors to include the risk of major adverse events.
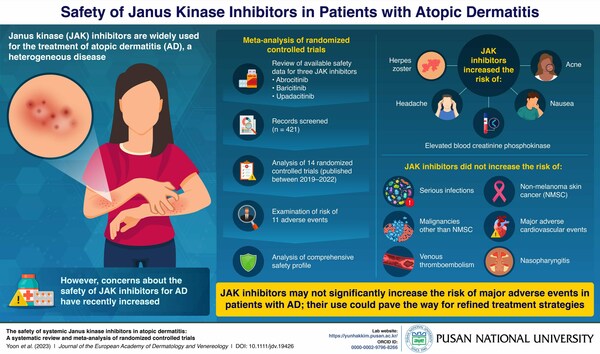
Researchers from Pusan National University in South Korea conducted an extensive analysis of randomized controlled trials (RCTs) involving JAK inhibitors in patients with AD to see whether the use of JAK inhibitors increased the occurrence of adverse events in patients with AD.
They identified studies reporting the adverse events associated with JAK inhibitor use from Medline, Embase, Clinicaltrials.gov, among others for this study. They reviewed the available safety data and analyzed the risk of 11 adverse events from 14 RCTs published between 2019 and 2022.
Their analysis revealed that the relative risk of developing herpes zoster, headache, acne, elevated blood creatinine phosphokinase, and nausea increased among those patients with AD who received treatment with JAK inhibitors. However, the incidence of serious conditions did not increase significantly in JAK inhibitors-exposed patients with AD.
“We believe that understanding and managing common adverse events is the need of the hour, particularly those like headache, acne, and nausea, among others,” says Associate Professor Yun Hak Kim of the University’s Department of Anatomy and Department of Biomedical Informatics, in a news release.
This analysis revealed a higher risk of acne in patients receiving upadacitinib and abrocitinib, and this increased in risk was dose dependent. Moreover, higher dose of JAK inhibitors was correlated with an increased risk of acne and elevated blood pressure.