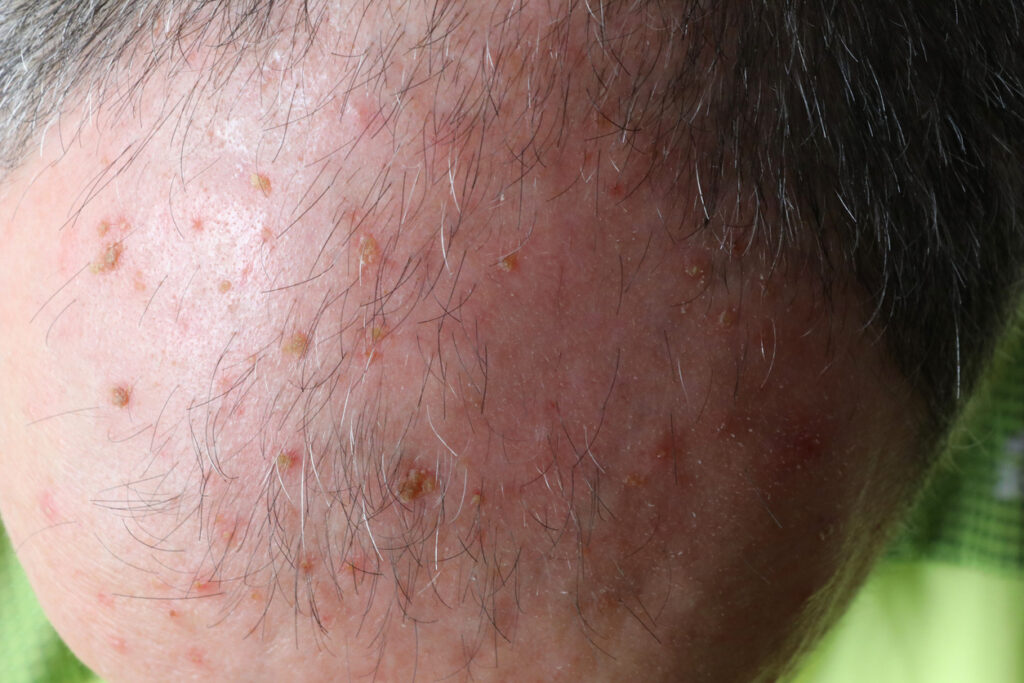
The U.S. Food and Drug Administration (FDA) has approved the next generation of Sun Pharma’s BLU-U Blue Light Photodynamic Therapy (PDT) Illuminator for treating actinic keratoses (AK).
The approval was granted under FDA’s Real-Time Review Program, which streamlines the review of applications for new drugs and biologics, particularly for oncology drugs, by allowing the FDA to access and review key data before the official application submission.
Uses LEDs Instead of Fluorescent Tubes
The new LED BLU-U features light emitting diode light (LED) panels as a replacement for the previous model’s fluorescent tubes. The new model (LED BLU-U), in combination with LEVULAN KERASTICK (aminolevulinic acid HCl) Topical Solution, 20%, is indicated for the treatment of minimally to moderately thick AK of the face, scalp, or upper extremities. LED BLU-U is approved for the same indications as the previous model but takes up less space in a dermatologist’s office, and has a more flexible five-panel shape, improved LED arrangement, lighter weight, and other updated functions that may increase patient comfort and ease of use.
“We are pleased to receive the FDA’s approval of LED BLU-U and look forward to seeing the positive impact this next generation device will have for those living with actinic keratosis,” says Abhay Gandhi, Sun Pharma North America CEO, in a news release. “As a company committed to innovation, we are confident that this new LED BLU-U model will provide improved efficiency and reliability while maintaining the safety and efficacy that healthcare professionals and people with AKs have come to trust from Sun Pharma.”
The LED BLU-U will be available for delivery in the near future. For details regarding availability, ordering, or implementation, contact your Sun Pharma Specialty Dermatology representative. For more information, healthcare professionals are encouraged to visit www.levulanhcp.com.